Article Text
Abstract
There is a significant unmet clinical need for a reliable point-of-care (POC) estimation of the blood haemoglobin (Hb) method. Current available methods, notably pulse oximetry, have certain limitations related to design and methodology of devices. These have low sensitivity for detecting serial change in the Hb values, especially at the lower range and are inaccurate in people with darker skin.
Objective This study aimed at developing a novel, non-invasive technology for the measurement of Hb and oxygen saturation.
Design This was an observational study.
Recruitment This was approved by the Institutional Review Board at the University of Texas at Arlington and 16 healthy adult volunteers (age 20–40 years) were recruited in this study.
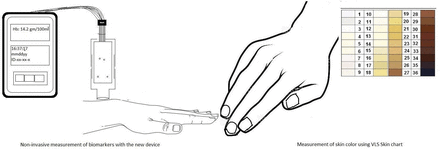
The investigational device (Shani) probe (United States Patent 11191460B1) consists of light emitting diodes with wavelengths ranging 520–580 nm, and a photosensor component. The probe is gently placed on the back of the subject’s wrist and reflected light is measured as an electrical signal, with digital recordings. Skin tone (or skin colour) was assessed by Von Luschan Chromatic Scale (VLS). Using a specific algorithm accounting for melanin (as determined from VLS Scale) and employing a software, the results can be displayed on screen as Hb values and ratio of tissue oxygen saturation.
Results The results of the investigational non-invasive (Shani) device were comparable with the invasive, point of care (POC) method (iSTAT, Abbott Inc.).
- emergency medicine
- primary healthcare
- hospital medicine
- public health
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is no correction for skin melanin in the present pulse oximeters using red-infrared light. This gives erratic readings in people with darker skin.
WHAT THIS STUDY ADDS
This is the first ever use of green light (520-580 nm) in non-invasive, hemoglobin and oximetry device. Skin melanin is quantified in this device and due corrections are made using a special algorithm. This eliminates the impact of skin color on the observations.
This one device can be potentially used in adults, children and neonates. This can be used in wearables and low-resource settings.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This device provides a reliable, non-invasive tool for haemoglobin and oxygen saturation monitoring in several settings (home, outpatient clinics, emergency room and intensive care units, and wearables). This eliminates racial disparity in these key biomarker assessments, with huge positive impact on outcomes in people of color, and low-resource countries.
Introduction
POC estimation of blood haemoglobin (Hb) and oxyhaemoglobin (O-Hb) concentration is commonly measured using the available pulse-oximetry or co-oximetry-based methods. Available pulse-oximetry technique estimates Hb and O-Hb saturation in the pulsating blood vessels of the fingertips (or earlobes). Blood oxygen saturation (SpO2) measured by pulse oximetry is currently used to monitor tissue hypoxia. However, these non-invasive spectroscopy-based methods are inaccurate in people with darker skin, likely due to an overlap in the absorption spectra of Hb and melanin.1–5
Severe anaemia is a major cause of hospital admissions and mortality in the developing world such as sub-Saharan Africa, especially in children.6 There is a significant unmet need in clinical practice in the region due to unavailability of an inexpensive, easy to use, non-invasive and accurate POC Hb method.7 POC Hb and O-Hb measurement methods are used in many clinical settings such as emergency triage, blood bank screening, perioperative care, and intensive care units, but the results are affected by tissue temperature, peripheral vasoconstriction, right heart failure, and skin colour.8–10 Emerging data indicate that pulse oximetry overestimates the SpO2 in people with darker skin. Reliance on pulse oximetry to triage patients and adjust supplemental oxygen levels may place dark-skinned patients at increased risk for hypoxaemia. This inaccurate assessment might delay timely interventions in the critical care setting. Emerging data suggest an association with higher mortality in dark-skinned individuals.11 12 This racial disparity is more evident during the recent COVID-19 pandemic with poor outcomes in dark-skinned patients compared with white patients.6 The United States Food and Drug Administration (FDA) has issued a public notice regarding the potential inaccuracies in oximetry measurements and cautioned its use in the clinical practice.2 3
Human Hb is a tetrameric metalloporphyrin. Haem part contains iron radicle and porphyrin. The globin part has two pairs of amino-acid chains. Healthy adults have ‘Haemoglobin-A’ made of two alpha and two beta chains [α2 β2] while a baby has ‘Haemoglobin-A [α2 β2]’ and ‘Haemoglobin-F, composed of two alpha and two gamma chains [α2 γ2]’.13 14 Absorption spectra of O-Hb shows different peaks than deoxyhaemoglobin. Absorbance spectroscopy, based on the Beer-Lambert law, is effective for measuring the quantity of a particular chemical in a given medium.15–17 The greatest region of dissimilarity between oxy and deoxyhaemoglobin is from 520 to 580 nm.15 18
With this, background of absorption spectrum of Hb in green light; we developed a new device for the non-invasive measurement of Hb using green light. This was done in two parts. In the first part, the absorption spectrum of Hb was studied in blood samples of adults and newborns.19 In the second part, these data were used in developing the new technology for measurement of Hb in healthy volunteers. Additionally, we developed a novel approach to account for the impact of skin melanin content on the estimation of blood Hb.
The investigational device probe emits a narrow beam of green light onto the skin, as further described below. Hb as well as the skin melanin absorb a portion of the incident light, due to their overlapping absorption spectra in the green (520–580 nm) wavelength range.4 Green light penetration is limited to approximately 2–3 mm depth from the skin surface.20 In contrast, red and infrared light used in the available pulse oximeter technology penetrates deeper.21 The cutaneous microcirculation is organised as two horizontal plexuses. One is situated 1–2 mm below the skin surface and the other is at the deep dermal–subcutaneous junction.22 The green light used in our device is suitable for the wrist sensor application as it penetrates enough to sense changes in the cutaneous capillary plexus, without interference from the deeper tissues and vessels.23 24 This allows estimation of total Hb, as well as estimation of oxygenation changes in the tissue capillaries with relative specificity.25 Interestingly, the absorption peaks of Hb in the green light spectra are identical for Hb-A (in normal adults), Hb-F (babies and patients with Beta-Thalassemia) and Hb-S (sickle cell anemia and trait). This has broad clinical implications, suggesting that this device can be used to detect and quantify concentrations of Hb in all these patient populations.26 27
In contrast, pulse oximetry uses LEDs in the red and infrared spectra with deep tissue penetration. The measured signals could be potentially affected by the deeply situated pulsating arteries. Thus, oxygen saturation measured by the current pulse-oximetry (SpO2) might not be very sensitive to detect minor early changes in tissue oxygenation (eg, tissue hypoxia) since arterial desaturation takes time. This problem could be magnified in people with darker skin, with additional interference from skin melanin.
Materials and methods
Investigational device
The investigational, handheld device (Shani) probe (United States Patent 11191460B1) consists of a probe housing the light source (LEDs) with wavelengths ranging 520–580 nm, and a photosensor circuitry. The light source shines light on the (wrist) skin of the subject (figure 1). After absorption by the skin melanin and Hb, a portion of the light is reflected. The optical sensor measures the amount of reflected light. The higher the Hb and or melanin, the greater the absorption and consequently less reflection of light (figure 1). The sensors are optically insulated and arranged in such that only the light reflected from the skin is collected by sensors. The reflected optical signals are converted to electrical current and displayed as a numerical value corresponding to blood Hb level.
Method of measurements.
The ratio of reflected light from the skin of subject’s wrist to reflected light from a standardised surface (reflectance standard) is measured as E1 (for LED1) and E2 (for LED2). A standardised laboratory made reflectance surface was developed after calibrating with Giga-Hertz 2% reflectance standard (Gigahertz Optik GmbH, 82 299 Tuerkenfeld, Germany). Sum of E1 and E2 is noted as E (total ratio).
Next, the validated Von Luschan Chromatic Scale (VLS) is used to assess the skin melanin content.12 VLS is an easy to use, clinical measure of the relative melanin content in the skin. It consists of numeric values from 1 to 36, with higher values representing more melanin content (darker tone) in the skin. VLS was assessed on the dorsal aspect of the wrist area by the same independent observer, in the same ambient (illumination etc) conditions. Using a specific algorithm accounting for melanin (as determined from VLS Scale and employing a software), results of total ratio or E may be displayed on screen as Hb values. By measuring relative concentrations of oxy and deoxy Hb values (by using calculations of E1 and E2), the device can display tissue oxygen saturation as well.
Clinical study
Sixteen healthy adult volunteers (age 20–40 years) were recruited in this study. After appropriate consent, the baseline demographic information was obtained.
The subject was asked to lie in the supine position. An intravenous cannula was placed in the right antecubital vein to obtain venous blood samples. These samples were used to estimate Hb (and venous oxygen content as well as saturation) by the invasive i-STAT (Abbott) method, used as a reference. Next, the baseline Hb observations were obtained using the investigational device, the commercially available pulse oximeter (Masimo Pronto, Masimo), and the invasive POC -Hb measurement method (i-STAT, Abbott).
The subject was then required to wear a heat jacket to increase the body temperature (the increment was up-to 1.5°F than baseline body temperature). Changes in body temperature simulate the clinically relevant fever setting.
A total of seven sets of observations (at approximately 10 min intervals) were obtained for each subject, using all three (investigational device, commercially available pulse oximetry and the invasive i-STAT) methods, over a period of 60 min. Means and SDs (of seven observations) for each method were calculated for every subject. The Hb observations estimated by the investigational device were compared with those obtained by the pulse oximeter, using i-STAT as the reference.
Results
The mean age of the study population was 24.1 (±0.8) years. There were 12 (75 %) males. There was a uniform skin tone distribution with approximately one-third (5/16) with fair skin, one-third (6/16) with brown skin, and one-third (5/16) with dark skin.
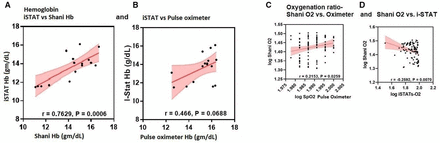
There was good correlation between Hb measured using i-STAT (Abbott) technology and Hb estimated by the investigational device (r=0.7629, p=0.0006; figure 2A). We observed poor correlation between the i-STAT measured Hb and pulse oximetry measured Hb (r=0.466, p=0.069; figure 2B).
(A, B) Correlation between iSTAT and pulse oximeter and Shani Device for mean haemoglobin values. (C, D) Correlation between (OR) measured by pulse oximeter, Shani Device and iSTAT. There was a strong correlation between the i-STAT device vs the Shani Hb device for the mean Hb values for each subject. (***p<0.0006) (A). The correlation for the i-STAT device vs the Pulse oximeter was not statistically significant (p=0.069) (B). There was positive correlation between the Oxygenation Ratio (Log Shani O2) measured by the investigational device and oxygen saturation (Log SpO2 Pulsat approximately 10 minutespute the OR using the dev intervals) were obe Oximeter). (*p<0.026) (C on the left). There was a strong negative correlation between the Oxygenation ratio (Log Shani O2) measured by the investigational device and the venous oxygen saturation (Log iSTATs-O2) mesured by the invasive i-STAT device (**p<0.007) (D on the right).
An algorithm was developed to compute the Oxygenation Ratio (OR) using the device-measured values (E1 and E2), and subject’s VLS skin tone to account for the impact of melanin. OR was defined as the relative change in the O-Hb concentration at the tissue capillary level (with respect to total hemoglobin Hb). We observed positive correlation between the investigational device-estimated OR and the pulse oximetry measured SpO2 (r=0.22, p=0.03; figure 2C). There was negative correlation between the investigational device estimated OR and regional venous oxygen saturation measured using the i-STAT (r=−0.26, p=0.007; figure 2D). Comparisons were done across the relative change in the oxyhemoglobin O-Hb concentrations as measured by the investigational device (ratio, Shani O2), oximetry saturation measured by the pulse oximeter, venous oxygen content measured by the i-STAT (mm Hg, pO2 mm Hg) and the venous oxygen saturation measured by the i-STAT (%venous saturation, i-STATs-O2). Logarithmic conversions were used for comparison to account for the variability across the scales.
Discussion
In 1972 Takuo Aoyagi, a Japanese electrical engineer developed the first non-invasive device for measurements of blood biomarkers—a pulse oximeter using red and infrared light. All of today’s oximeters are based on Mr. Aoyagi ‘s original principles of pulse oximetry. Certain limitations of this technology are apparent during the recent COVID-19 pandemic. Notably, the measurements obtained using the pair of red and infrared LEDs are interfered by the skin melanin content.
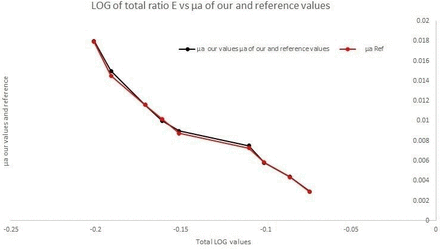
We have developed a new device for non-invasive measurement of hemoglobin Hb and OR while addressing these limitations. Aoyagi is often recognised for putting the ‘pulse or life’ in pulse oximetry. We would like to make a similar analogy that we have added ‘colour’ to this technology. During the prior preclinical development of our device, the principle of reflectance spectrometry was verified in tissue phantom studies using horse blood, in different concentrations. Further validation of the device was done by measuring coefficient of absorption of blood - μa- at 550 nm wavelength using different concentrations of horse blood. Our results were similar to the reported literature (figure 3).
Validation of Shani device in tissue phantom studies.
As described above, the investigational device shows promise for non-invasive estimation of total Hb. Intrasubject variability was significantly low. The oximeter failed to record Hb on two occasions due to cold extremities in one instance and very dark skin colour in the other, but investigational device was able to estimate Hb in both cases.
Increased oxygen demand at the tissue level (due to temperature change, for example) leads to augmented blood flow. This increase in the amount of O-Hb is expected to increase the relative O-Hb content at the tissue capillary level. As the oxygen extraction in the tissue increases, the relative concentration of O-Hb in the draining veins would show a decline, as reflected by the lower (regional) venous oxygen saturation. Thus, this novel technology can be used in combination with the pulse oximetry for better monitoring of the tissue level oxygen exchange.
Strengths of our study include the sizeable number of observations in subjects with diverse skin melanin content. Data integrity was ensured by real time electronic recording of all measurements, with timestamps. The limitations of the study include a relatively small number of healthy subjects. Larger studies involving patients with a variable degree of hypoxia are needed for further development and validation of this device.
To summarize, our device uses reflection spectrometry and targets the superficial part of skin and capillaries for measurement of tissue oxygen status.22 Measurement of tissue oxygen status is more important than pulse oximetry, because several studies have reported that decreases in SpO2 detected by pulse oximetry may lag behind the actual clinical event.28 Second, when light is passed through skin, in addition to being absorbed or reflected, some gets scattered. However, simulation studies have shown that within the 520–580 nm region, the error in measurements by not accounting for scattering of light is very small.29
Our technology offers an additional advantage to overcome the impact of skin colour (melanin) during the estimation process. We have validated our algorithm to account for melanin effect on Hb measurements. One device can be used in all age groups from newborns to elderly. Since it is battery operated and portable, it is highly suitable for remote monitoring. The current device prototype has a specific geometric design. The technology can be embodied into wearables or other prototypes. The data are securely stored and can be transferred electronically.
Conclusions
Our preliminary findings support a potential new technology for the assessment of Hb and capillary oxygen. The technology and the device are non-invasive and could potentially overcome current limitations of the pulse-oximetry devices.
Further clinical development of the device would include both preclinical and clinical validation across a variety of settings. The currently available non-invasive pulse oximeter could potentially be used as a predicate for laboratory and clinical comparisons. The United States FDA has issued specific guidance with respect to approval requirements for non-invasive devices using various regulatory strategies.30
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Institutional Review Board (IRB) at the University of Texas-Arlington. A written, informed consent was obtained from each participant. STU-2021-0150 Part-1:- IRB Approval Date: 02/23/2021 Part-2:- IRB Approval Date: 05/11/2021. Copies of consent forms are with IRB authorities.
Acknowledgments
We thank Dr. Michael Nelson, Associate Professor, Department of Kinesiology, University of Texas Arlington for allowing the use of Kinesiology Laboratory for the clinical study.
References
Footnotes
Contributors SGG conceptualised and designed the study, drafted the initial manuscript, monitored the site, approved the final manuscript as submitted and is the guarantor. VD drafted the initial manuscript, and approved the final manuscript as submitted. GA provided the scientific support and guidance. All authors have accepted responsibility for the entire content of this manuscript and approved its submission. The data were analysed using IBM SPSS Statistics for Windows, version 25 (IBM Corp) and GraphPad PRISM (version 9.1, GraphPad Software, California, USA).
Funding This research was funded by Shani Biotechnologies, LLC, Austin, Texas.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.