Article Text
Abstract
Introduction Healthcare expenses are reaching unaffordable levels worldwide and reverse innovation could play a role in decreasing these expenses and improving healthcare accessibility. The ReMotion Knee, a prosthetic knee primarily developed for low-income countries, could serve as a reverse innovation for people with a lower limb amputation. This study aimed to evaluate the ReMotion Knee as a potential reverse innovation in high-income countries, specifically in terms of functional mobility.
Methods Nine participants with a transfemoral amputation or knee exarticulation were included in this randomised crossover trial. The ReMotion Knee was compared with the participants’ current prosthetic knee in terms of functional mobility and subjective experiences. The primary outcome in this study was the L test for functional mobility. Secondary outcomes were additional clinical performance tests and subjective experiences (balance confidence, walking comfort, test performance and fatigue).
Results Participants scored significantly better using their current prosthetic knee than using the ReMotion Knee on primary outcome, the L test (p<0.01, median difference 7.5 s, IQR 6.1–10.6) and all secondary outcomes except experienced test performance and fatigue. All participants were able to safely perform all clinical tests with the ReMotion Knee, even after a short familiarisation period.
Conclusions The ReMotion Knee has the potential to become a reverse innovation after modifications improving velocity, walking comfort and weight limit. Collaboration between high-income and low-income countries can facilitate further development of the ReMotion Knee and could result in alternative products and treatments that could reduce healthcare costs while still providing a good quality of care.
Trial registration number NCT04700085.
- amputation, traumatic
- health care economics and organizations
- quality of health care
- rehabillitation
- global health
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available on request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- amputation, traumatic
- health care economics and organizations
- quality of health care
- rehabillitation
- global health
WHAT IS ALREADY KNOWN ON THIS TOPIC
Reverse innovations provide an opportunity to reduce healthcare costs while still providing a good quality of care, but there still is little research available on lower-priced or decremental cost-effective assistive medical devices.
WHAT THIS STUDY ADDS
This study was a first attempt to evaluate an assistive device, the $80 ReMotion Knee, as a reverse innovation in high-income countries.
The ReMotion Knee allowed users to safely perform key clinical performance tests for functional mobility, even after a short familiarisation period, but the current standard of care prosthetic knees still showed better outcomes.
HOW THIS STUDY MIGHT IMPACT RESEARCH, PRACTICE AND POLICY
The ReMotion Knee has potential as a reverse innovation in high-income countries after improving walking velocity, walking comfort and increasing the current body weight limit of 80 kg.
More research evaluating potential reverse innovations, such as the ReMotion Knee, could lead to a decrease in healthcare expenses in high-income countries and improve healthcare accessibility in worldwide.
Introduction
Healthcare organisations worldwide aim to improve the health of populations, improve patient healthcare experience and reduce the costs of care per capita.1 More advanced medical technologies are continually being developed that improve health and healthcare experiences,2 3 but these also result in higher product or treatment costs and thus rising healthcare expenses.3–6 Cost pressures from medical innovations combined with an ageing population form a substantial threat to the long-term sustainability of healthcare systems worldwide.7 In the last decade, health leaders in high-income countries have become interested in reverse innovation.8–12 Reverse innovations are products or ideas that are introduced in developing countries before being adopted in high-income countries.13 These products are often affordable durable, require little training and easy to use. Moreover, they are designed to function and survive in extreme and/or unpredictable conditions. Unfortunately, low-income country of origin products are still generally perceived to be less reliable and less safe. Furthermore, the term reverse innovation may raise prejudices about these products. The term falsely implies that they are inferior to products in high-income countries, which is not supported because they are rarely researched as alternatives to the current standard of care within the western medical sector.3 6 Therefore, reverse innovations provide an opportunity to contain or even reduce healthcare costs in high-income countries while still providing a good quality of care.2 3 12 14 Lower-limb prosthetics form a typical example of a medical field with increasing technological developments that lead to higher costs.15 16 New prosthetic knees are continually being developed and marketed at higher prices. Furthermore, the more advanced—and expensive—prostheses are being adjusted to meet the needs of a large group of less active users. The rising prices of prosthetics combined with the increase in incidence of lower limb amputations17 will further raise the costs of prosthetics care.18 Reverse innovation could play a role in controlling these costs in the decades ahead. So far there has been little research on lower-priced or low-income country of origin prostheses and this topic therefore deserves more attention.
In 2008, The JaipurFoot Organization together with the university of Stanford developed the first version of the ReMotion Knee: an $80 prosthetic knee specifically designed for the amputee population and environment in low-income counties. With the ReMotion Knee, they aimed to provide an affordable, simple, durable, easy-to-use and all-terrain prosthetic knee to people who could not afford the current prosthetic care. Since then, it has been developed further and has been successfully implemented in 33 countries and is now being manufactured on a larger scale (Equalize Health, San Francisco, California, USA). The Remotion Knee was primarily designed as a frugal innovation, fulfilling 6 of the 10 core competencies of frugal innovations (ruggedisation, lightweight, having a human centric design, simplification, new distribution models and affordability).19 However, the ReMotion Knee may also have potential as a reverse innovation and thereby presents an opportunity to reduce healthcare costs among amputees in high-income countries as well. However, it has only been evaluated in terms of overall satisfaction among users20 and has not been evaluated within a high-income setting yet.
By our knowledge, this study is the first attempt to evaluate reverse innovations in assistive devices where advances in technology have led to increased healthcare costs in recent decades. We compared the ReMotion Knee to the mechanical prosthetic knees that are currently used in high-income countries on the most important health outcomes for people with a lower-limb amputation: functional mobility, walking comfort, balance and balance confidence.21 We hypothesised that the participants would be able to perform key functional clinical tests using the ReMotion Knee, but that the test outcomes would be slightly better with their current prosthetic knee.
Methods
Design
This exploratory study uses a randomised crossover trial design with two conditions: the ReMotion Knee and the participants’ current prosthetic knee. Participants performed a set of clinical tests with both prosthetic knees in a randomised order. Measurements were carried out at the Sint Maartenskliniek (Ubbergen, the Netherlands) between February 2019 and August 2019. All participants signed an informed consent form prior to study participation and provided consent of participation and use and publication of the collected data.
Participants
Participants in this study had experienced a unilateral transfemoral amputation (TFA) or knee exarticulation (KE). Inclusion criteria were age ≥18 years, Medicare Functional Classification Level (MFCL) 2 or 3 (most probable users of the ReMotion Knee), ≥1 year since amputation and currently using a mechanical prosthetic knee with a primarily polycentric design. Exclusion criteria were increasing stump pain with activity, >20° hip flexion contracture, inability to stand and walk for 30 min, body weight >80 kg (weight limit of the ReMotion Knee), no universal socket connection and an osseointegrated prosthesis. Participants were approached by their treating prosthetist (OIM Orthopedie, Nijmegen, the Netherlands). If interested, they received an informational letter and 1 week later they were contacted by the researcher for their inclusion or exclusion.
The ReMotion Knee
The ReMotion Knee is a mechanical polycentric prosthetic knee that was developed for use in low-income countries. It has a lifespan of 3–5 years, which is comparable to other mechanical knees currently used.22 However, it does not include a hydraulic or pneumatic system. The ReMotion Knee provides basic functional mobility and costs only $80, thereby being very low priced compared with standard mechanical knees used in the Netherlands, which typically cost between $1.000 and $8.000. It has received a CE mark and has been approved as complying with ISO 10328 standards.
Blinding
Blinding of both the researcher and the participants was not possible. However, as price can influence preference,6 no information about the ReMotion Knee was given other than that it was a newly developed knee type comparable to the participants’ own prosthetic knee. Furthermore, it is known that country of origin can affect perception.23 Therefore, this information was withheld from the participants as well.
Outcome measures
The primary outcome measure was functional mobility, which measured with the L test. This includes standing up from a chair, walking 20 m with four turns (L shape) and sitting down.24 Individual differences between the knees were compared with the minimal clinically important difference (MCID) of 4.5 s25. Secondary outcome measures were balance (Berg Balance Scale (BBS)26 and body weight distribution27), precision stepping (Four Square Step Test (FSST)28) and advanced walking tasks (modified Emory Functional Ambulation Profile (mEFAP)29). Furthermore, subjective experiences of balance confidence, fatigue, test performance and walking comfort were measured using a numeric rating scale (NRS 0–10). Finally, walking speed, step length and %single limb support time were primarily defined as outcome measures as well, but were excluded due inaccurate step detection of the Inertial Measurement Units used for measurements.
Measurement procedure
Prior to the measurements, participants filled out a general characteristics questionnaire, the ABC-scale questionnaire for balance confidence30 and the SIGAM-WAP questionnaire for mobility level.31 They also scored their perceived exertion on a Borg Scale (6–20).
Subsequently, participants started measurements with either the ReMotion Knee or their current prosthetic knee (determined by randomisation). The measurement procedure included: (1) standing on a scale (first with both feet, then with the prosthetic foot only), (2) the L test, (3) the FSST, (4) the BBS and (5) the mEFAP. The L test and FSST were performed twice and the best of the two attempts was used. After these clinical tests, participants scored their experienced fatigue, balance confidence, performance and walking comfort with the tested knee (NRS 0–10). They were then asked which aspects of the tested knee they would like to see improved. After the first condition, the prosthetic knee was switched by the prosthetist who followed the user manual of the manufacturer for correct alignment of the ReMotion Knee. Meanwhile, participants rested until their Borg Score was equal to or one point higher than before the start of the first condition, with a minimum of 15 min rest to minimise the effect of fatigue on the test results. Subsequently, the measurements were repeated with the second prosthetic knee.
All participants received a 30-min familiarisation session with the ReMotion Knee under the supervision of a physical therapist during which they practiced standing, transfers, walking, turning and climbing stairs. All these tasks had to be performed safely before starting the measurements. The familiarisation session was held just before the ReMotion Knee measurement condition and the 15-min resting period took place after familiarisation and before the measurements.
Sample size determination
No comparable research with this primary outcome measure was available. Previous studies on the comparison of prosthetic knees have shown differences in other functional outcome measures using samples sized between 10 participants and 22 participants for within-subject comparisons.32–36 Therefore, our sample size was set at 20 participants (10 with MFCL 2 and 10 with MFCL 3). However, the inclusion was terminated after nine participants, because there were no more clients at OIM Orthopedie who were interested in and eligible for participation (especially due to the weight limit of 80 kg of the ReMotion Knee). Based on non-parametric statistical testing methods and an expected decrease larger than the MCID of 4.5 s25 for the primary outcome in all participants, at least six participants were considered sufficient for statistical significance. Unfortunately, the initially set secondary objective of this study, to examine the effect of MFCL on the functional changes with the ReMotion Knee, could no longer be pursued with this small sample size.
Randomisation
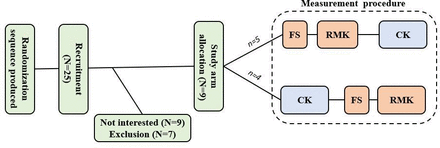
All participants performed the clinical tests under two conditions: once with the ReMotion Knee and once with their current prosthetic knee. The order of the conditions was predetermined by the researcher before the start of inclusion using block randomisation (allocation ratio 1:1) to ensure an equal number of participants started with the ReMotion Knee and with their current knee (figure 1). Furthermore, this design was chosen to reduce the possible influence of fatigue on the results.
Randomisation, recruitment procedure and allocation of the participants in crossover study design. CK, current knee; FS, familiarisation session; RMK, ReMotion Knee.
Statistical analysis
Descriptive statistics were used to summarise participants’ characteristics. A two-tailed Wilcoxon signed rank test was used to examine the within-subject difference between the ReMotion Knee and the current prosthetic knee for both the primary and secondary outcome measures. The alpha level for significance was set a priori at 0.05, without correction for multiple testing. The statistical analysis was performed using IBM SPSS Statistics 25 (IBM, Chicago, Illinois, USA).
Patient and public involvement
No patients were involved in the concept, design or recruitment plans for this study, because there were very little potential participants and participants had to be blinded from any information about the ReMotion Knee. One patient was involved in a pilot measurement to test the feasibility of the procedure.
Results
Nine participants with a unilateral TFA (n=7) or KE (n=2) were included in this study. Their characteristics are presented in table 1. Five of the nine participants started the measurements with the ReMotion Knee and four started with their current prosthetic knee. A total of 25 clients were approached by OIM Orthopedie; nine were not interested in participation and seven were excluded based on the exclusion criteria (figure 1).
Demographic information relating to the participants
Clinical performance tests
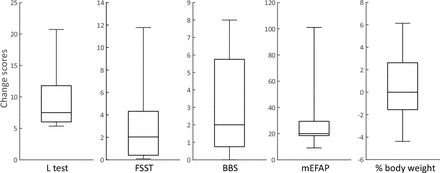
The participants scored significantly better on the L test (median difference=7.5 s, r=−0.63, p=0.008), FSST (r=−0.63, p=0.008), BBS (r=−0.56, p=0.018) and mEFAP (r=−0.63, p=0.008) with their current knee than with the ReMotion Knee (table 2). The difference on the L test was larger than the MCID of 4.5 s for all participants. There was no significant difference body weight distribution during static stance (r=−0.16, p=0.499). Change scores on the clinical performance tests are shown in figure 2.
Clinical performance test results
Boxplot of Individual change scores on clinical tests. Individual change was computed as (value ReMotion Knee−value current knee). An individual change >0 indicates a higher value for the ReMotion Knee than the current knee. BBS, Berg Balance Scale; FSST, Four Square Step Test; mEFAP, modified Emory Functional Ambulation Profile. Central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. Whiskers extend to the most extreme data points.
Subjective experiences
Participants experienced better balance confidence (r=−0.56, p=0.017) and walking comfort (r=−0.60, p=0.011) with their current prosthetic knee (table 3). There was no difference in experienced fatigue (r=−0.10, p=0.667) and test performance (r=−0.30, p=0.196). The suggestions most frequently mentioned for improvement of the ReMotion Knee were associated with the swing phase velocity of the shank (mentioned four times), the resistance to flexion during stance (mentioned three times) and the extension damping at terminal swing (mentioned two times).
NRS scores for subjective experiences
Discussion
In this first study evaluating a potential reverse innovation in assistive devices, the ReMotion prosthetic knee was compared with the mechanical prosthetic knees currently used in the Netherlands in terms of health outcomes. We hypothesised that the participants would be able to perform key functional clinical tests using the ReMotion Knee, but that the test outcomes would be slightly better with their current prosthetic knee. The most important health outcomes for people with a lower-limb amputation, functional mobility, walking comfort, balance and balance confidence21 were significantly better using the participants’ current knee than using the ReMotion Knee. The difference in the primary outcome measure (L test) with the ReMotion Knee was larger than the MCID of 4.5 s in all participants.25 However, balance tasks were less affected by knee type than walking tasks. For example, only two of the nine participants showed a decrease in BBS larger than the minimal detectable change.36 Finally, participants experienced equal fatigue and test performance with both knee types. Although there were differences between the knees in favour of the participant’s current knee, it should be noted that all participants were able to safely complete all the performance tests with the ReMotion Knee.
Recommendations for further development
The ReMotion Knee in its current form may have difficulty competing with the standard of care mechanical knees in high-income countries. The differences found in this study can mainly be attributed to the prosthetic knee design, as the participants’ current knees have a hydraulic or pneumatic control system and the ReMotion Knee does not. A pneumatic system enables more variation in walking speed, a higher maximum walking speed,37 better stability during stance and stepping37 38 and better walking comfort. Nevertheless, all participants were able to perform all clinical tests with the $80 ReMotion Knee. This suggests that the ReMotion Knee could have potential as a reverse innovation. However, to have the ReMotion Knee compete better with the currently available prosthetic knees, we recommend further developments in order to provide more walking comfort and to support higher walking velocity. Additionally, increasing the weight limit of the ReMotion Knee is essential, as the current weight limit of 80 kg does not match the demographics of the western amputee population and is very likely to negatively influence its applicability in western healthcare. While these changes will increase the price somewhat, it is still not expected that it will reach the price level of the mechanical knees that are currently used. Furthermore, new low-cost hydraulic knees are already being developed39 that may provide these benefits too37 38 and it is possible that the function and comfort of prosthetic knees designed for low-income countries will increase to the level of those in high-income countries within the next decade. Furthermore, introducing these potential reverse innovations to the western market, producers will likely have to overcome a negative country of origin effect/bias which could influence their new customers’ perception of the product.40 If they do, these innovations have the potential to enter high-income markets as affordable alternative prosthetic knees, possibly with a better price-quality ratio.
Limitations
Our study had several limitations. First, the acclimatisation period was quite short and measurements were performed only in a controlled environment. This approach was chosen because this was the first study evaluating the Remotion Knee in western healthcare and there was no information about safety or fall incidence prior to this study. For follow-up research, it is recommended that participants have a longer familiarisation period, preferably in the home situation. Second, although very limited information was provided about the ReMotion Knee, the participants could not entirely be blinded to the prostheses. The change of knee type in itself has probably influenced the performance and most of all the perception of safety. The short acclimatisation time and lack of blinding probably worked in favour of the participants’ current knee. Therefore, it would be interesting to examine if comparable results would be found among participants that currently use the ReMotion Knee. Third, the sample size was small, but because of the strong significant differences found it is expected that the inclusion of more participants would not have resulted in different outcomes. The within-subjects design also reduces the chance of individual differences influencing the results and requires a smaller sample size compared with a between-subject design. Fourth, the prosthetic knee and foot currently used were not standardised and varied between participants. However, the results are still considered generalisable because all knees had similar mechanisms and all participants used the same prosthetic foot in both conditions. Finally, this was an exploratory study on functional mobility and did not evaluate cost-effectiveness. To get a better impression of the usability of the ReMotion Knee, future studies should also consider additional costs (eg, prosthetic adjustments and adverse events) and evaluate the cost-effectiveness in various healthcare systems within western society.
Innovation in the field of prosthetics
In our current healthcare economics system, new products are priced based on their added value for patients and the pricing of comparable products.6 Within the prosthetics market, the ReMotion Knee is on one side of the innovation spectrum. It provides basic functionality and has the potential to make a large impact by providing affordable care for as many people as possible worldwide.11 21 On the other side of the spectrum, there are the technologically advanced microprocessor-controlled knees (MPKs), known to improve walking comfort,41 safety41–43 and balance confidence.44 While primarily designed for the highest active users, they are now being adjusted to meet the needs of a much larger group of less active users as well. Unfortunately, their high prices greatly limit accessibility to a wider public, both in high-income and low-income countries. This distinction clearly shows that there are different types of innovation in prosthetic care—one providing basic care to many and the other providing optimal care to only a few. Comparing the impact of the ReMotion Knee in low-income countries with the differences found in this study and the added value of the MPK, one can wonder whether the price gaps of around $2.000 (ReMotion vs current knees) and around $15.000 (mechanical knee vs MPK) are proportional to the improvement in functionality gained with these knees. Therefore, a reverse innovation of MPKs is highly recommended to meet the needs of people with a lower-limb amputation in high-income countries.
Future of reverse innovations
Healthcare expenses are now reaching unaffordable levels worldwide and medical innovations are becoming increasingly expensive, limiting their accessibility and leading to growing healthcare inequalities.6 This problem of unaffordable care, rising costs and limited accessibility requires a shift in focus in research and innovation towards controlling healthcare costs while ensuring a good price–quality ratio. This will improve the quality of life of many around the world instead of just helping the few who are affluent.5 10 In low-income countries, affordable alternatives are already being developed. Examples are the subject of this study (the ReMotion Knee), the Aravind Eyecare Hospital, the Indian Supraflex SES coronary stent and the MACi portable ECG machine produced by GE Healthcare.9 Instead of bringing knowledge and downgraded products to low-income countries, high-income countries should explore new ideas and products that might initially not be noticed or accepted because of their price or country of origin. Country-of-origin associations can negatively influence the consumers’ perception of a product and thereby affect the consumers’ willingness to buy, or even lead to product avoidance.40 Therefore, developers should be aware of a possible negative bias towards their products in their product positioning strategy. Nevertheless, comparing more reverse innovations with the current standard of care, as was done in this study, could better reveal their potential for high-income countries. This requires greater collaboration between high-income and low-income countries and openness to learning from less familiar healthcare systems, as well as other industries.45 Ultimately, we expect that the reverse innovations will have substantial growth potential and can thus contribute to a more affordable care and a more sustainable healthcare economics system worldwide.
Conclusion
The ReMotion Knee has the potential to become a reverse innovation, as basic functional mobility is achieved with his prosthetic knee. However, for its successful implementation in high-income countries, modifications improving velocity, safety, comfort and weight limit are recommended. Collaboration between high-income and low-income countries could facilitate this and could lead to the development of other alternative products and treatments that will reduce healthcare expenses while still providing a good quality of care.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available on request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was conducted according to the declaration of Helsinki (Brazil, 2013), approved by Medical ethical committee of Arnhem-Nijmegen, the Netherlands (study number 2018-4795, NL-number NL67281.091.18). Participants gave informed consent to participate in the study before taking part.
References
Footnotes
Contributors All authors contributed to the study concept and design. Data acquisition was performed by WvO and data analysis was performed by WvO and NLWK. All authors contributed to the interpretation of the data. WvO drafted the work. All authors revised it critically, gave final approval of the version to be published and are accountable for all aspects of the work. WvO is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.