Article Text
Abstract
The incidence of fatal anaphylaxis is significantly higher among young people aged 15–25 compared with other age groups. Hospital admission or fatal outcome following anaphylaxis often results from failure to adhere to an agreed anaphylaxis action plan (AAP). The main barriers for adherence include lack of confidence to recognise symptoms of severe reaction, lack of confidence and skills to correctly use an adrenaline auto-injector (AAI), and not having the AAI available when needed. We describe the development of a smart phone application (app) to increase young peoples’ adherence to AAP. The development of the app was informed by information from a literature review to identify factors enhancing and impeding young peoples’ adherence to their AAP, combined with data from consultations with intended users and clinicians working with young people at risk of anaphylaxis regarding their needs and expectations with regard to the content and technical features of the app. The design process was underpinned by the novel Behavioural Intervention Technologies model. This ensured that the apps’ content is evidence based, complies with current guidelines, and responds to users’ needs and preferences in relation to content and technical characteristics. ‘Anaphylaxis’ app is the first smart phone app that comprises a comprehensive personalised AAP. Since its launch in February 2013, it has been downloaded by approximately 16 000 users worldwide. Further research is required to demonstrate its effectiveness in improving self-management of anaphylactic risk in young people.
- Medical Apps
- mHealth
- Inventions
Statistics from Altmetric.com
Introduction
Young people between 15 and 24 years of age pose higher risks than any other age group for life-threatening allergic reactions1 with over 50% of deaths due to food-induced anaphylaxis occurring in this age group.2 Failure to inject epinephrine is the most common reason for hospital admission or fatal outcome following severe allergic reaction in young people.3 ,4 A recent review concludes that the main reason for delaying or failing to use an adrenaline auto-injector (AAI) by young people is being unsure whether the symptoms are severe enough to be considered life threatening and thus require injecting epinephrine.5
Independent management of severe allergies by young people involves ensuring that the young person has the knowledge and skills to: (A) minimise risk of severe allergic reaction through avoiding allergens and (B) follow an agreed anaphylaxis action plan (AAP) to recognise symptoms of severe reaction early on, and reduce its negative impact by initiating appropriate treatment (usually AAI).
People at risk of anaphylaxis generally agree their personalised AAP with their healthcare provider and receive a paper copy of it during the consultation. They usually receive instruction on how to use prescribed AAI and have an opportunity to try it out using a training device. Providing young people with a written AAP does not guarantee adherence.6 To promote adherence an alternative, more user-friendly way of providing APP was needed. Smartphone applications (apps) are becoming increasingly popular in delivering health behaviour change interventions to wide audiences.7 The 2013 Ofcom Report indicates that 75% of adolescents and young adults in the UK own a smartphone.8 In this age group, socioeconomic status does not influence the likelihood of owning a smartphone.9 Thus, a smartphone app was considered the best technology to deliver AAP and maximise its availability for the young people who are at the highest risk of fatal anaphylaxis.
App development process
A robust strategy for developing m-health interventions is to review the current literature to specify the content and theoretical basis of a prototype app, which is then tested for acceptability with the target users.10 Ideally the two processes are conducted iteratively, with the revisions being done at each stage in response to users’ feedback. This approach informed the development of the ‘Anaphylaxis’ app to ensure that its content is evidence based, complies with current guidelines, and responds to users’ needs and preferences in relation to content and technical characteristics.
To ensure that the development process is systematic and replicable, it was underpinned by a novel Behavioural Intervention Technologies (BITs) model.11 BITs are described as “behavioural and psychological interventions that use a broad range of technologies, such as mobile phones, the Web, and sensors, aimed at changing behaviours and cognitions related to health, mental health, and wellness”.12 The BITs model is illustrated in the development process below in relation to questions why, how, what and when. Our process comprised the following steps:
Rapid literature review to identify factors facilitating and hindering young peoples’ adherence to AAP.
Based on findings from literature establishing aims the app is designed to achieve (WHY).
Selecting behavioural strategies with evidence for effectiveness in increasing adherence to treatment in young people with chronic conditions corresponding with each aim (HOW).
Designing elements of the app to be used to deliver selected behavioural strategies (WHAT).
User based design: A qualitative study with intended users, and clinicians working with young people at risk of anaphylaxis to find out:
If the proposed aims of the app address barriers for young peoples’ adherence to AAP (clinicians) and respond to young peoples’ needs for support to effectively manage emergency situations (users).
If proposed elements of the app are feasible and acceptable for users.
What technical characteristics of the app are preferred by users.
Amending design document in response to clinicians’ and users’ feedback.
Designing prototype ‘Anaphylaxis’ app.
Identifying factors promoting and hindering adherence to AAP, and establishing aims for the ‘Anaphylaxis’ app (WHY)
A Delphi study involving a 25-person, multidisciplinary expert panel achieved consensus on the key components of an AAP including: awareness of trigger factors (100%), recognition and emergency management of reactions of different severity (100%), and clear information on epinephrine use (100%).13 In the first instance, we reviewed research to identify factors facilitating and impeding young peoples’ adherence to key components of the AAP.
Knowledge and confidence to correctly identify symptoms of severe allergic reactions usually determine what further actions are taken.13 Recognising signs of severe reaction is necessary to initiate an AAP; however, correct recognition of symptoms does not guarantee that a person will follow the agreed emergency procedures.14 Young people report that they are often unsure when anaphylaxis symptoms they are experiencing are severe enough to be considered life threatening and thus require injecting epinephrine. Since there are no warning symptoms that can reliably signal evolving anaphylaxis, and there is no clearly defined threshold above which symptoms become life threatening, patients have difficulty deciding whether they should use AAI, and as a result often delay it or decide that injection is not necessary.5
Young people often lack confidence that they can correctly use AAI, especially if they received AAI training sometime ago.15 ,16 Many young people prefer to take alternative medication or visit hospital rather than self-inject.17 Studies show that the likelihood of carrying AAI is inversely proportional to the time since the last severe episode.18 Many do not carry their AAI at all times because they are conspicuous and make them feel ‘different’ from peers.19
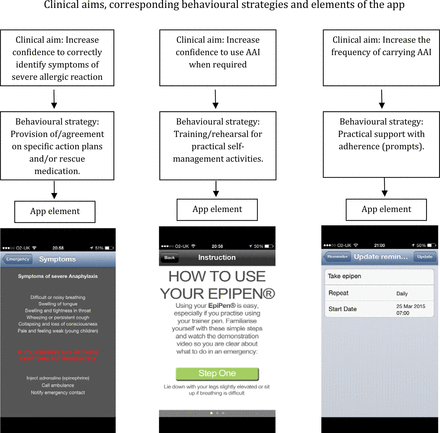
The overall aim of the ‘Anaphylaxis’ app was to increase adolescents’ adherence to AAP. Based on the above research, three specific objectives were established: (1) to increase adolescents’ confidence to correctly identify symptoms of anaphylaxis; (2) to increase adolescents’ confidence to use AAI when required; (3) to increase the frequency of carrying AAI.
The app does not cover trigger avoidance as there are other apps designed solely for that purpose (eg, Allergy Guard, Allergy Scan, iAvoid Food Allergy, Can I Eat It?).
Selecting behavioural strategies corresponding with each aim of the ‘Anaphylaxis’ app (HOW) and elements of the app designed to deliver each strategy (WHAT)
To describe strategies corresponding with each aim (HOW), we applied the research-based Taxonomy of Self-Management Support Components.20 The selection of strategies corresponding with each aim of the app was underpinned by research on the effectiveness of behavioural strategies in increasing adherence to treatment in young people with chronic conditions.
Currently there are no studies that would identify strategies to increase patients’ confidence to recognise symptoms of anaphylaxis. However, it is important that patients understand that even if initial symptoms are mild, there is a significant potential for rapid progression to a severe, possibly life-threatening reaction.14 Since it is often impossible to predict the ultimate severity of anaphylaxis, injecting epinephrine is recommended as soon as symptoms, however mild, are recognised,21 especially since unnecessary administration does not pose any serious health risk.21 There is strong evidence for the role of action plans in increasing adherence to treatment and promoting correct response in emergency situations,22 so we proposed using this strategy to facilitate recognition of a severe reaction. Providing a written action plan to young people with asthma significantly increased patients’ adherence to medication and correct response in emergency situations,23 reduced exacerbations, emergency department visits and hospitalisations.20 In accordance with current clinical guidelines, and to simplify the decision process, the proposed action plan indicates that recognising any of the listed symptoms of anaphylaxis should result in prompt epinephrine administration.
There are no studies on the effectiveness of providing instruction how to use AAI correctly. However, there is evidence that competency in using AAI decreases as time elapses from the first instruction,16 and regular auto-injector training promotes correct injection technique and increases comfort of using AAI.16 To increase young peoples’ confidence to correctly use AAI, we proposed a strategy facilitating AAI training providing video and/or written instruction how to use AAI as prescribed. It provides an easy to follow step-by-step instruction for emergency situations.
A review of literature of interventions to increase adolescents’ adherence to treatment of chronic conditions supported the use of goal setting and prompts to increase the regularity of taking prescribed medications.22 A recent systematic review of the effectiveness of electronic reminders indicates they are effective in improving adherence to treatment in patients with chronic conditions.24 Thus, we provided practical support with adherence (prompts) to increase the frequency of carrying AAI (table 1).
‘Anaphylaxis’ application design process informed by BITs model
A qualitative user-based design study
The user-based design study25 aimed to determine: (A) if the proposed aims of the app address barriers for young peoples’ adherence to AAP and respond to young peoples’ needs for support to effectively manage emergency situations, (B) if the proposed elements of the app are feasible and acceptable for users, (C) what technical characteristics of the app are preferred by users.
Methods
The study (Coventry University ethics approval: ref. P9256) was advertised on the Anaphylaxis Campaign UK website between January and April 2012. Potential participants contacted the lead researcher to go through the consent process. Participants received via email a document describing the proposed aims, elements and technical characteristics of the app, and were asked to consider its acceptability, usefulness and user friendliness to discuss it during the interview. Semistructured interviews lasting on average 45 min were conducted over the phone by the lead researcher (health psychologist).
A focus group lasting 35 min with four clinicians working with young people with severe allergies was arranged to elicit their views about factors that enhance and impede young peoples’ adherence to AAP, and the support they need.
Interviews and focus group were recorded, transcribed and analysed thematically.26
Results
Interviews with users
Participants
Five males and five females with a mean age 18.1 years (range 15–20 years) were interviewed and sample saturation was achieved. All respondents were in education, four lived with parents. All were diagnosed with food allergies, prescribed an AAI and previously experienced severe allergic reactions requiring use of AAI on two or more occasions.
Findings
Intention to use app for convenience
All participants stated that having AAP in their phone would be a convenient way to ensure that they always have it available. A female (age 16) said: “I have a sheet of paper from my doctor with all information about my allergy but I never really take it with me. It is too big to put in my purse. Having it in my phone is a good idea”.
App features valued
All proposed aims and elements of the app were considered valuable. Nine respondents stated it would be very useful if the app could help them determine when they should use their AAI. A male (age 18) said: “My doctor gave me the list of symptoms and told me if I have any of them, I should inject. I never carry it with me though(…) I can sort of remember but I am never really sure if I need to inject”. All users said that having a detailed instruction for how to use their AAI would be particularly important. A female (age 15) said: “I am always worried that I won't remember how to use my injector when I really needed it(…) An instruction I can follow would make me feel more confident that I will do it right”. Seven participants stated that having reminders to take their AAI would be helpful. A male (age 17) said: “When I am in a hurry, I often forget to put the ‘pen’ in my bag. If something was reminding me every morning just before I leave, I would be more likely to carry it”.
Usability of features
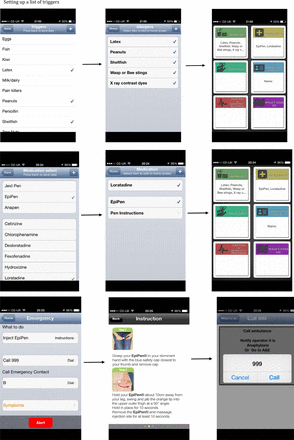
All users wanted to minimise the complexity of the app to maximise the convenience of using it and reduce time needed to set it up. Most users preferred simple icons, basic font and minimal amount of graphics to simplify using the app. All users agreed that the option of setting up their personalised profile including a list of triggers, medications and emergency contacts would be desired options.
Focus group with clinicians
Participants
Two paediatric allergy consultants with over 15 years of experience and two paediatric nurses working in allergy clinic for over 7 years participated in the focus group.
Findings
Barriers for effective anaphylaxis management
All clinicians agreed the biggest barrier for effective anaphylaxis management is not using AAI when needed. An allergy consultant said: “Most of A&E admissions could be avoided if patients used their auto-injectors. I often see adolescents who ended up in the hospital still having it in their pocket”.
Barriers for using AAI
Two clinicians said that many adolescents and/or their parents are often not sure if the injection is needed. A consultant said: “Patients often wait till very last minute before they inject and it is usually because they are not sure whether their reaction will develop to be serious or can they get away with not injecting”. Not knowing the correct technique was considered another barrier. A nurse said: “During every visit I ask patient and parent to demonstrate using the auto-injector and 8 out of 10 starts off wrong”. Not carrying AAI at all times was considered to be a problem among older adolescents as for younger ones the responsibility was usually the parents’. A consultant said: “(…) The problem starts when they turn 15 or so, and start going out with friends, without their injector”.
Value of the app
All clinicians agreed that an app to support adherence to AAP can be a very useful tool. A consultant said: “Young people use their phones for everything. If all this information is in it, they may actually use it when they need it. When they have a reaction they often have nothing to refer to immediately, and being able to look up symptoms or follow an instruction to inject will increase the odds of them doing it right”.
Conclusions
Information gathered through interviews with intended users and clinicians working with young people at risk of anaphylaxis regarding barriers for adherence to AAP was consistent with findings from literature. Lack of young peoples’ adherence to AAP mainly results from lack of confidence to recognise symptoms of severe reaction and correctly use AAI, and not having AAI available when reaction occurs. The app addresses all three problems through applying behavioural strategies with proven effectiveness to improve the likelihood of performing actions leading to better outcomes (ie, adherence to AAP; see figure 1). The app also has functions suggested as useful by users: a personalised list of known reaction triggers, current medications (including AAI linked to a video/written step-by-step instruction how to use it), emergency contacts that can be speed dialled without exiting the app, ‘near me’ function linked to a map and navigation to find the nearest emergency hospital and pharmacies, and ‘alert’ button to attract attention in an emergency situation (see figure 2).
Description of ‘Anaphylaxis’ application's (app's) clinical aims, corresponding behavioural strategies and elements of the app designed to achieve the aims.
Anaphylaxis' application (app) screenshots presenting steps to set up the app (list of triggers and medication) and instructions in case of emergency.
Discussion
This paper describes the process of developing a novel smartphone app for young people at risk of anaphylaxis designed to increase adherence to AAP. There are some limitations that need to be considered regarding potential for effectiveness of the app in achieving its targets. First, studies show that young people up to the age of 24 are at significant risk of fatal outcome following severe allergic reaction.1 ,2 During the development process, we did not consult users over the age of 20, and it is possible that older users would have different expectations that the app is currently not addressing. We also made an assumption that the app will be used not only by young people at risk of anaphylaxis, but also by others (eg, parents, peers and teachers), in case they need to assist a person experiencing severe reaction. However, people other than clinicians were not involved in the app development process. We may assume the AAP within the app will be relatively easy to follow for a person who had some previous experience of assisting someone experiencing severe reaction, though we do not know whether it would be of any use for a person unfamiliar with anaphylaxis symptoms and emergency procedures. To establish how acceptable and useful the app is for people over the age of 20, and people who are not allergy sufferers and have no previous experience of dealing with severe reaction, further usability studies are required with purposefully selected subsamples.
Studies show that for young people with severe allergies, their condition is often a source of social difficulties.19 Some tend to hide their condition by eating potentially unsafe foods, not carrying AAI when they are out with friends, or if they are experiencing a reaction, hide from others and wait for it to subside on its own, rather than alert peers and seek medical help.27 Addressing the problem of communicating about anaphylaxis with others would require a more complex intervention and it would not be feasible to deliver it as a part of the app.
Being able to consult the app when experiencing an allergic reaction could help young people to correctly decide when they should use AAI and reduce the risks of hospitalisation and potentially fatal outcome. Having a detailed instruction on how to use AAI could increase confidence to self-inject, but if others administer the injection it is more likely to be completed correctly. The ‘Anaphylaxis’ app cannot replace education and collaboration between patients, parents and healthcare providers to ensure that the young person is ready to assume responsibility for independent anaphylaxis management, it could however be a useful tool to increase adherence to AAP and promote correct use of AAI. Further research is needed to establish whether the app is useful for young adults and people without previous experience with anaphylaxis, and whether it is effective in increasing confidence in behaviour to reduce the risk of, and consequences of, anaphylactic shock.
Currently the ‘Anaphylaxis’ app is available only for Apple devices. It is free and can be downloaded from https://itunes.apple.com/gb/app/anaphylaxis/id583861393?mt=8&ls=1. In the nearest future, we are planning to offer the ‘Anaphylaxis’ app to suers of other mobile operating systems including Android and Windows Phone.
References
Footnotes
Collaborators Dr Andrew Clark, Cambridge University Medical School; Ms Lynne Regent, Anaphylaxis Campaign, UK.
Contributors JKA lead the development of described ‘Anaphylaxis’ app, designed, conducted and analysed results of qualitative study which was a part of the development process, drafted the initial version of the manuscript, addressed issues raised by the coauthor (LMW) after reviewing the manuscript, and approved final version submitted for publication. LMW contributed to the development of the app (advising on methodology), revised initial and subsequent versions of the manuscript and approved final version submitted for publication.
Funding The development of the app was funded internally by Coventry University HEIF4 grant (£2K).
Competing interests None.
Patient consent Obtained.
Ethics approval Coventry University Ethics.
Provenance and peer review Not commissioned; externally peer reviewed.