Article Text
Statistics from Altmetric.com
- Surgical Wound
- Surgical Procedures, Operative
- Wound Infection
- Health Care Quality, Access, and Evaluation
What are the new findings
Parallelised, iterative development of individual elements of a prototype technology may enable frugal as well as highly optimised innovation with increased, ongoing user feedback. However, managing integration risk, such as via stepwise integration, is critical to overall success of the innovation.
The SurgiField system is able to provide both passive and active barriers against intraoperative contamination during a broad range of surgical procedures, particularly the so-called bellwether procedures that are used internationally to benchmark adequate access to surgical care.
A modular, surgical site-based enclosure could be made efficient to deploy and take down, ergonomic and compatible with typical surgical field access requirements. This could be implemented with a conveniently sized package similar to large surgical drapes used normally for any major surgical procedure.
How might it impact on healthcare in the future
Our work implements a paradigm shift from controlling the surgical environment at the operating theatre level to controlling it more precisely at the surgical site level. This conceptually expands the possibilities for systems and technological innovations needed to increase access to safe surgical care where patients need it.
SurgiField systems could help expand access to safe surgery at the point of need.
Introduction
In conditions ranging from pneumothorax to acute compartment syndrome to fractures to traumatic and degenerative joint disease to appendicitis to obstructed labour to postpartum haemorrhage; safe, timely surgical intervention is the deciding factor among recovery, mortality or long-term disability. Consequently, access to quality surgical care is a global priority across the resource spectrum. Yet despite this, an estimated 18 million people die annually from conditions that safe surgery could address. Even more experience permanent disability from delayed, inaccessible, or unsafe surgery, generating a gross domestic product loss of US$820 billion from lost annual productivity.1 Far from requiring resource-intensive, sophisticated surgeries, the vast majority of surgical needs globally are captured by the simple, so-called bellwether surgical procedures.2
Surgical care has traditionally relied on consistent availability of three resource categories: (1) functional equipment and materials, (2) appropriate facility space and (3) medical staff.3 4 Given the practical challenges of disseminating these resources consistently in many austere settings, including but not limited to conflict settings, disaster zones, low-to-middle-income countries and rural regions; surgical care availability is often limited to centralised referral settings.5 Even higher-resource settings regard cost-containment and quality improvement as ongoing challenges to care equity and sustainability. For instance, Californian operating theatres cost an average of US$37 per minute to run.6
High equipment and facility costs are driven in part by maintaining the sterility of the entire operating theatre throughout the surgical procedure, regardless of the number of personnel and activity. This paradigm requires every single surgical team member to wear extensive personal protective equipment, while sophisticated ventilation systems continuously run filtered air through the entire theatre. By contrast, the critical surgical site—the area of the body that is open and vulnerable to contamination—is never more than a few square feet, a tiny space within the massive theatre.7
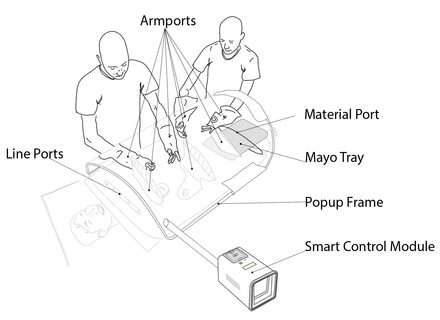
Here, we report the development and implementation of a new paradigm, in which sterility is focused around the surgical site itself. The SurgiField system consists of a single-use sterile medical device, the SurgiBubble. SurgiBubble drapes over the patient, isolating the surgical site with a physical barrier from the surrounding environment while allowing aseptic access via armports, a material port and lineports. Everything and everyone outside of the SurgiBubble (including most of the patient’s body) is prevented from contaminating the surgical site by a passive physical barrier as well as active airflow. For the latter, the SurgiBubble is inflated with filtered air by a battery-powered Smart Control Module (SCM). A (non-sterile) Popup Frame (PuF) provides additional structural integrity.
This paper describes the iterative design process and testing through which this system has been developed. We then share the outcomes and key learnings of this design process. We conclude by considering the implications of this innovation on the provision of surgical care.
Methods
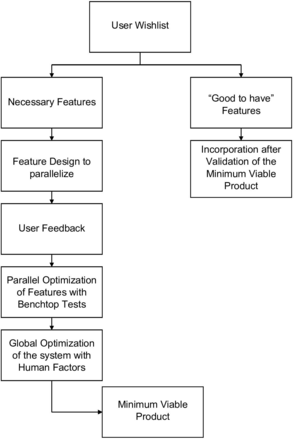
The design methodology for the SurgiField system is an iterative one centred on user needs. These steps are summarised in figure 1:
Gather user requests for device as possible inputs to device design from a range of medical practitioners.
Categorise essential and non-essential design inputs by cross checking medical safety standards and having users rank their own requests.
Create device design specifications, equivalent to a target product profile, using essential design inputs.
Validate technological features through benchtop testing. More details in ‘Benchtop Testing’ paragraph.
Perform iterative human factors/usability testing. Details are provided in ‘Human Factors Testing’ paragraph.
Assess, reevaluate and update design at each stage of human factors/usability testing.
Freeze design as minimum viable product once essential design inputs are incorporated and validated.
Non-essential design inputs are noted and to be considered for subsequent product versions.
Design methodology flow chart.
User device requests were generated by crowdsourcing from different stakeholders, that is, humanitarian surgeons, military surgeons, logistics experts and other clinical team members working in over a dozen countries. Multiple discussions with stakeholders generated the following list of essential features for this device:
Essential design components
Filtered air.
Active airflow.
Access to the surgical site for practitioners and surgical equipment.
Clear visibility of surgical site.
Easy to set up and use.
Appropriate for ‘Bellwether procedures (caesarean delivery, laparotomy and treatment of open fractures)’2 and fasciotomies.
Electricity independent.
Safe for users and patients.
Easy to transport to remote locations.
Able to accommodate between one to seven individual hands
Non-essential design components
Independent lighting.
Compatible with robotic and laparoscopic surgery.
Applicable for use in extreme heat/cold conditions.
These procedures were selected for three major reasons. First, they are all high-frequency but relatively resource-efficient procedures across a broad range of settings. Second, they imply a surgical system’s ability to setup for and support interventions on different anatomical areas. For instance, if there is visibility and range of motion to support a four-compartment fasciotomy, there would also be visibility and range of motion to support smaller procedures like deep debridement in the same area. Third, they all typically require wide as well as deep exposure including into initially clean or clean-contaminated areas.
Once device specifications were defined, an iterative parallel design with user feedback and the optimisation of each element was initiated. Benchtop tests were performed to validate the local optimisations. Global optimisation on a system level was performed and validated through human factors tests with surgeons and other medical providers.
Benchtop testing
Several of the essential design inputs (access to the surgical site, visibility of the surgical site, ease of use) ensured that the SurgiBubble did not impede the surgical procedure. Benchtop studies were performed to optimise the following elements:
Size:
SurgiBubble size is a small form factor that does not impede the movements of the medical professionals.
Visual clarity:
Material for bubbles is visually clear, antistick, tear resistant and physically light. Visual clarity and use are validated during human factor testing.
Ports integration:
Ports provide access into the sterile system and are designed for entry of arms, instruments or lines without creating air leaks.
Sleeve/arm ports configuration:
Arm ports allow surgical team to pass instruments.
Donning and doffing without compromising the SurgiBubble structural integrity.
Line ports:
Line ports are designed to be self-sealing to preserve structural integrity of the system while easily accommodating surgical lines inside the sterile environment. Line ports are validated by inserting different lines and verifying the absence of air leaks.
Material Ports:
Material port is sized optimally to insert and remove a surgical tray during procedures, and potentially remove newborns post caesarean section. A magnetic sealing mechanism closes the port. Validation is performed by opening the port, inserting/removing surgical trays multiple times and then sealing the port while verifying that there are no leaks.
Limb port:
Limb port is for the insertion of limbs into the surgical environment subsequent isolation from the exterior environment (no leaks). Validation is performed by testing the insertion of different mannequin limbs.
Smart Control Module
The SCM provides stable, filtered airflow to the surgical site.
Filtration is verified through leakage and airflow tests to ensure all airflow out of the SCM passes through the filter.
Firmware optimisation is used to maximise battery life.
Human factors testing
The purpose of human factors tests is to allow potential users to use the SurgiField product and provide feedback on the device pertaining to the instructions for use, usability and potential use cases. All human factors testing was conducted with medical staff who were qualified to either assist with or perform the simulated surgical intervention.
This input was then used to streamline the instructions and update the design as needed to fit user needs. Human factors testing of different stages occurred in a few different settings: at the prototyping spaces, at various meeting room spaces with portable surgical mannequins, and at a mass casualty and humanitarian emergency simulation. The latter was a military medical trainee event operated by the United States, and consisted of outdoor simulated deployment over 2 weeks with a mix of patient mannequin and actor simulations.
The standard human factors test ran as follows:
User sets up the SurgiField system.
User turns on the airflow system and inflates the bubble.
User mimics motions pertinent to possible surgeries, including general arm motions in the surgical area, placement and use of a surgical tray and instruments in the enclosure. An assistant user hands off instruments and assists in the simulated surgical field.
User takes off the SurgiField system.
User is evaluated via a survey and subsequent semi-structured interview.
The final SurgiField design is that of a glovebox-like-drape with multiple access ports, the SurgiBubble, which providers stick onto the patient. This is inflated and its internal environment controlled by a battery powered control module, the SCM. An exoskeleton, the PuF, provides additional structural integrity (figure 2).
The SurgiField kit.
Results
Design outputs
The SurgiField system is the result of a comprehensive data and risk driven design process that ensures its safety and efficacy.8–11 Numerous surgical team members ensure that the SurgiField system addresses the particular needs of patients and users. Each generation of the device’s performance has been verified via the peer-reviewed benchtop studies referenced above. Key parameters from the benchtop studies include:
Ultraportability: SurgiField fits into a 30L backpack and weighs less than 5 kgs. Packaged SurgiBubble measures 23 cm wide and 45 cm long.
Ergonomics: From views of the surgical field to headlamp compatibility to arm range of motion to interpersonal instrument handoff, the SurgiBubble has been extensively designed and tested to accommodate a large range of user and patient morphologies.
Cleanliness: The SurgiBubble is a sterile device, and the SCM delivers HEPA filtered air to the surgical field.12
Applicability: Compatible with Bellwether Procedures implying compatibility with abdominal and orthopaedic procedures.
Discussion
Design process findings
The single biggest challenge in the design process was the creation of sufficient and pertinent system specifications given a lack of similar prior product for comparison. Also, no readily available target product profile existed. Yet many stakeholders, particularly surgical users, simultaneously wanted a leapfrog solution for their accessibility and safe surgery needs, and wanted minimum disruption to existing workflows. To optimise for these conflicting demands, all system specifications had to be created through iterative design and testing cycles with user input.
New problems arose during integration testing, when the various independently manufactured components of the SurgiField system were tested for compatibility with each other. For example, during early iterations, the PuF stretched the SurgiBubble material sufficiently to affect the electronic system’s airflow calibration parameters to inflate the SurgiBubble. Serial system reoptimisation was needed to enable compatibility in this and other cases. Integration was a high priority going into final design and manufacturing of device components.
It was especially valuable for us to expand our search for user input beyond the surgeons to also include other key members of the surgical and perioperative teams, such as anesthesiologists. We learnt to optimise the system to fit not just surgeons’ workflow, but also the broader teams', from initial patient setup to anaesthesia induction to invasive monitoring. Seemingly small things like how a surgical team might negotiate whether a subclavian central line site should be kept inside or outside the enclosure, how the anaesthesia team could access and troubleshoot an epidural if needed, how certain configurations of the system might affect an intubated versus non-intubated patient, etc proved critical to optimise.
Incorporating diverse interests in safe surgery from diverse stakeholders and sources of initial design needs input. It became important to differentiate essential and non-essential user inputs by stakeholder type, and to prioritise accordingly to achieve an impactful minimum viable solution. Three main metrics were used in the following order: minimisation of possible risk to users and patients, applicable regulatory needs, and manufacturability.
Manufacturing costs and future considerations
While advanced prototype-level production has been expensive due to the unique design, we have mapped out pathways to drive down the cost rapidly with economies of scale in production as well as streamlining the manufacturing process. In parallel, we have established cost-effective, robust supply chains for critical materials and components through key partnerships.
A key insight from early-stage stakeholder interviews and prior market research was that ‘similar products’ are perceived differently by stakeholders, none of which directly compare with the SurgiField system. The most cited alternatives included semiportable laminar airflow systems or operating suites mounted in vehicles and temporary buildings. SurgiBox addresses the limitations of these existing solutions by eliminating high upfront and maintenance costs, providing multilayered protection, reducing external dependencies such as electricity, and markedly increasing portability.
Because this innovation offers a premium quality improvement—with a more-precisely defined and controlled microenvironment inside the enclosure than state of the art, yet can be efficiently applied and removed to allow rapid patient turnaround—we plan to achieve economies of scale by first offering SurgiField systems in higher-resource settings requiring more efficient access to surgical care, for example, fasciotomies in emergency, field, and bedside settings, before expanding to lower-resource settings. While it would be impossible for any product to be cheaper per se than to use no intraoperative protection at all, the global initiatives to expand access to safe surgery are increasingly highlighting the hidden systems and societal costs of unsafe surgery.13 14 This deployment and scaleup plan will also enable the innovation to achieve sustainability.
Future and ongoing work on this project include refinement of the manufactured system through field testing and deployment/scaleup planning. We are currently working on obtaining regulatory approval from relevant health authorities.
Conclusion
Surgically treatable conditions cause more death and disability than AIDS, tuberculosis and malaria combined.1 Thus, increasing access to high-quality surgery is critical, through investment and innovation in the three pillars of: functional equipment and materials, appropriate facility space and medical staff.
The SurgiField system provides passive and active barriers against intraoperative contamination during a broad range of surgical procedures, particularly the Bellwether procedures that are used internationally to benchmark adequate access to surgical care. This work demonstrates the feasibility of a paradigm shift from controlling the surgical environment at the operating theatre level to controlling it more precisely at the surgical site level. This paradigm shift conceptually expands the possibilities for systems and technological innovations needed to increase access to surgical care where patients need it.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Massachusetts General Hospital Institutional Review Board approved the final human factors testing. The remainder of the testing reported in this study adhere to typical biodesign norms with benchtop testing and ad hoc testing, which do not typically require Ethics or IRB approval. Participants gave informed consent to participate in the study before taking part.
Footnotes
Twitter @DrToTheRescue, @miketeod, @HassanMashbari, @OsaidesserMD, @AnderDorken
Contributors AD-G conducted human factors testing and needs analysis. AMa conducted needs analysis. AMo helped lead field simulation testing and conducted needs analysis. DLT helped lead iterative prototyping, needs analysis, human factors testing, and benchtop testing; and led revisions of this manuscript. DRK helped lead design of human factors testing and needs analysis. HM conducted human factors testing and needs analysis. MNC led needs-analysis and policy analysis. MHMT led design of benchtop testing and early-stage project management. OA conducted human factors testing and needs-analysis. RDS conducted and helped design human factors testing. RJS conducted needs-analysis and iterative prototyping. SB conducted needs-analysis and human factors testing. AR conducted electronics and integration testing; JG cowrote the manuscript. MK conducted human factors and benchtop testing. DF supervised benchtop testing. SJ conducted needs-analysis and electronic testing; and led writing of this manuscript. All coauthors read and had the opportunity to revise the manuscript.
Funding The work described has been funded by Grand Challenges Canada R-HGC-POC-1904-24565, US DOD Small Business Innovation Research FA-8649-20-9-9067 and Wellcome Trust 215989/Z/19/Z.
Competing interests All coauthors have worked with and/or work with Project SurgiBox. DLT, SJ, MHMT, MK, HM, RJS and DRK hold minority stakes in SurgiBox. SJ, AR, MK and JG receive financial compensation for their work with SurgiBox. SB, HM, AMo, DF and MNC serve as advisors to SurgiBox.
Provenance and peer review Not commissioned; externally peer reviewed.