Article Text
Abstract
Introduction Determination of blood lactate levels supports decision-making in a range of medical conditions. Invasive blood-sampling and laboratory access are often required, and measurements provide a static profile at each instance. We conducted a phase I clinical study validating performance of a microneedle patch for minimally invasive, continuous lactate measurement in healthy volunteers.
Methods Five healthy adult participants wore a solid microneedle biosensor patch on their forearms and undertook aerobic exercise for 30 min. The microneedle biosensor quantifies lactate concentrations in interstitial fluid within the dermis continuously and in real-time. Outputs were captured as sensor current and compared with lactate concentrations from venous blood and microdialysis.
Results The biosensor was well-tolerated. Participants generated a median peak venous lactate of 9.25 mmol/L (IQR 6.73–10.71). Microdialysate concentrations of lactate closely correlated with blood. Microneedle biosensor current followed venous lactate concentrations and dynamics, with good agreement seen in all participants. There was an estimated lag-time of 5 min (IQR −4 to 11 min) between microneedle and blood lactate measurements.
Conclusion This study provides first-in-human data on use of a minimally invasive microneedle patch for continuous lactate measurement, providing dynamic monitoring. This low-cost platform offers distinct advantages to frequent blood sampling in a wide range of clinical settings, especially where access to laboratory services is limited or blood sampling is infeasible. Implementation of this technology in healthcare settings could support personalised decision-making in a variety of hospital and community settings.
Trial registration number NCT04238611.
- diagnosis
- clinical decision-making
- biomedical engineering
- patient care
- infectious disease medicine
Data availability statement
Data are available upon reasonable request. Data is available upon request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Summary box
What are the new findings?
We developed a lactate microneedle patch to continuously measure interstitial fluid lactate in real-time and validated this in human volunteers.
The patch is applied onto the skin surface without need for specialist equipment and readings closely follow venous lactate measurements up to 13 mmol/L.
The microneedle patch was well tolerated by all participants and no adverse events were reported.
How might it impact on healthcare in the future?
This device could support clinical decision-making and bypass need for blood testing in hospital and community healthcare settings in the future. It would be of particular benefit in special patient groups such as children.
Introduction
Raised lactate concentrations in blood are associated with all-cause mortality in hospitalised patients.1 The dynamics of lactate change over time and higher rates of early clearance are also associated with favourable responses to therapy and improved clinical outcomes.2 Lactate levels in humans result from the physiological interplay between tissue perfusion, hepatic and renal clearance, tissue hypoxia and the rate of glycolysis.3 Use of lactate as a biomarker to guide medical therapy and risk-stratification has been extensively validated, and supports management of infections such as sepsis,4 malaria5 and dengue,6 as well as in trauma,7 acute heart failure,8 interoperative optimisation9 and exercise training.10
In acute clinical settings, blood lactate concentrations are commonly quantified using laboratory analysers. Availability of bedside point-of-care measurements through blood gas analysers and capillary lactate devices further improves access to testing by reducing turnaround time. However, contemporary measurement methods all require blood sampling: venous puncture or expert arterial puncture are uncomfortable and can lead to complications, and the poor concordance of capillary lactate with whole blood restricts its use in clinical settings.11 Venesection poses particular challenges in special populations such as neonates or children, and the timely analysis of blood samples is often not feasible in healthcare settings with limited access to laboratory services.12 Where frequent measurements of lactate are clinically indicated—repeated sampling may be facilitated through placement of an arterial catheter and implantable intravenous continuous sensors have also been proposed.13 These invasive interventions are not possible beyond critical care settings and therefore preclude the role of frequent lactate monitoring as an adjunct to decision-making in prehospital, community healthcare or resource-limited settings.
Recent developments have supported minimally invasive sampling of a range of bodily fluids. Analysis of interstitial fluid (ISF) in particular is promising—as the primary constituent of extracellular fluid, the compartment exists in dynamic equilibrium with plasma.14 Relationships between blood and ISF lactate in pathology are complex, and have been described in hospitalised patients using microdialysis, an invasive ISF sampling technique.15 16 In a cohort of patients with sepsis in intensive care, changes in ISF lactate levels preceded changes in blood lactate, suggesting the former could serve as a sensitive and early marker of pathology at the local tissue level.17 ⇓
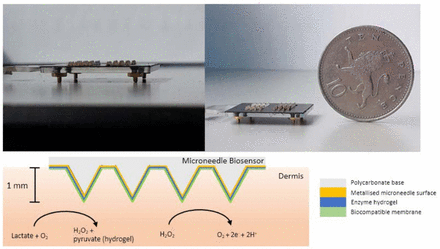
Measurement of substrates within the ISF is enabled through the use of minimally invasive platforms such as the microneedle biosensor.18 The small device consists of a plastic base with arrays containing 1 mm protrusions, with each array acting as individual biosensors. When the microneedle biosensor is placed on skin surface, these protrusions are in continuous, direct contact with ISF within the viable epidermis and dermis. The electrochemical detection of lactate is mediated through an enzyme-based sensing biocompatible hydrogel layer (see figure 1). An electrical current at the microneedle surface is measured and results can be displayed in real-time. As the microneedle protrusions in the skin lie superficial to the nerve layer, pain and discomfort is also minimised.19 Clinical studies using the microneedle platform have demonstrated good performance and comfort in prolonged usage for up to 24 hours in glucose monitoring20 and penicillin monitoring.21
The microneedle biosensor measures less than 2×2 cm and consists of small 1 mm protrusions which penetrate the stratum corneum in the epidermis to come into direct contact with tissue interstitial fluid (ISF) (top left and right). Lactate in the ISF is converted to pyruvate and hydrogen peroxide, with the latter being oxidised at the biosensor electrode surface, which is held at +0.7 V versus Ag|AgCl reference electrode (bottom). The resulting current to ISF lactate concentration.
We hypothesise that a minimally invasive continuous lactate biosensor built on the microneedle platform could offer distinct patient and operational benefits resulting in improved clinical management in the healthcare setting: real-time continuous measurements are likely to directly inform clinical decision-making. We therefore conducted a first-in-human Phase I clinical study evaluating the performance of the microneedle-based lactate biosensor in healthy volunteers. Aerobic exercise was used as a proxy means of increasing body lactate concentrations. We determined ISF lactate concentrations using the microneedle biosensor and comparison with lactate levels obtained in venous blood. In order to characterise the relationship between venous and ISF lactate in exercise, microdialysis was used to provide a reference measurement.
Methods
Study design
This was a phase I clinical pilot device study in healthy volunteers. The objective was evaluation of microneedle biosensors in measuring continuous ISF lactate in real-time before, during and after a short period of moderate aerobic exercise.
Patient and public involvement
Use of the microneedle patch has shown to be acceptable by patients and the public.22 The specific microneedle platform used in our study has been showcased at public engagement events, science festivals and scientific meetings by our group. As an alternative to blood testing the microneedle patch has high acceptability (median Likert score 9/10).23 We incorporated an end of study questionnaire evaluating attitudes towards the platform and plan to disseminate results to the public and consult with patient representatives in inform design of the next phases of the clinical study.
Participants
Between 16 February 2021 and 10 July 2021, participants were identified through recruitment posters placed around Imperial College London advertising the study. Male and female adult (18 years or older) healthy volunteers with no significant medical history, who exercised regularly at moderate intensity for at least 30 min two times a week were eligible. Details of study design, and recruitment are presented in online supplemental appendix 1.
Supplemental material
Microneedle description and fabrication
The microneedle patch consists of a polycarbonate base measuring 2×2 cm consisting of protrusions which sit less than 1 mm into the dermal layer of the skin. The patch is connected by wires to a potentiostat which provides real-time readout of electrical current. A lactate oxidase enzyme layer in the microneedle results in a current proportionate to ISF lactate concentration. Details of the lactate microneedle patch, fabrication and statistical analysis are presented in figure 1 and online supplemental appendix 2.
Study procedures
Single-use lactate microneedle biosensors were placed on inner forearm skin surface cleaned with 2% chlorhexidine gluconate/70% isopropyl alcohol and applied using firm thumb pressure for 60 s. The sensor was left in-situ on the forearm for 60 min for stabilisation. The participant was then asked to cycle on an exercise bicycle (Ergoselect 200, Ergoline Germany) at 60 rpm at power increments of 35 W up to a maximum of 210 W, for a total of 30 min according to the protocol followed by a rest period of 30 min. The exercise bicycle used in our study provides variable resistance so the participant cycling at a fixed rate of 60 rpm will produce the target power output. As the goal was for exercise to take place at moderate intensity, the maximum power output was dynamically adjusted on an individual basis according to participant preference and/or at the discretion of the researchers. Venous lactate was sampled at regular 5 min intervals and processed within 12 hours at a UKAS-accredited laboratory through an Architect Ci8200 analyser platform (Abbott, USA). A visual analogue score in a questionnaire was administered to the participants after 2 hours of microneedle placement. Consent for microdialysis was obtained only from one participant (number 5) and therefore performed only for that individual. A feedback questionnaire was given to all participants at the end of the study.
Results
Eleven individuals responded to the study advertisement. One was excluded because of pre-existing health conditions and five persons agreed to proceed to in-person screening. These five participants were enrolled into the study between 19 May 2021 and 13 July 2021 and assigned study numbers. One participant consented to undergoing microdialysis. The median age was 32 years (IQR 27–33), and one (20%) participant was female. All participants completed 30 duration minutes of active exercise with a median power output of 113 W (IQR 93–130 W). The median baseline pre-exercise venous lactate was 1.40 mmol/L (IQR 1.23–1.52), peak venous lactate was 9.25 mmol/L (IQR 6.73–10.71) and venous lactate at the end of the resting period was 2.41 mmol/L (IQR 2.06–2.90). There were no adverse events reported during the study. Characteristics of the participants are shown in table 1.
Characteristics of the five participants enrolled
Microneedle performance
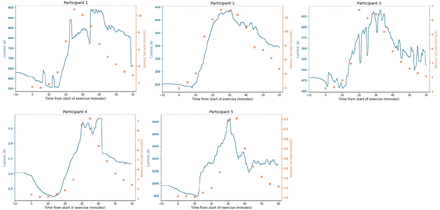
Microneedle biosensor current was plotted against time alongside venous lactate for each participant and shown in figure 2. There were no significant changes in the biosensor current during the initial stabilisation phase. There was a subsequent rise and fall in venous blood lactate during the exercise (0–30 min) and resting (30–60 min) phases, respectively. Continuous microneedle biosensor current followed venous concentrations closely over time. For participants 1–3, significant increases in biosensor current followed rise in venous lactate but for participants 4 and 5, there was increase in biosensor current before measurable rises in venous lactate. We observed different patterns of concordance between biosensor current and venous lactate for all participants between the exercise and resting phase: with greater lag in biosensor change with respect to venous lactate during the resting phase, suggesting slower ISF lactate clearance in skin compared with blood.
Microneedle biosensor current (blue continuous), venous lactate sampling (orange crosses) against time for individual participants (n=5). Exercise commenced at 0 min and stopped at 30 min, followed by a rest phase until the end of the study at 60 min.
Microdialysis
Microdialysis was performed in participant 5 to understand trends in ISF lactate concentration and to provide a reference measurement between biosensor current and blood lactate (figure 3). In general, there was a positive association between dialysate and venous lactate concentrations up to 5 mmol/L during exercise and rest, as well as between dialysate lactate concentration and biosensor current seen. ISF lactate concentration was significantly lower compared with that of blood for different reasons including variable recovery of lactate inherent to the microdialysis technique.
Venous lactate plotted against dialysate lactate concentrations from microdialysis (left). Blue squares represent the exercise phase and the orange triangles represent the rest phase of the study. Green crosses represent dialysate lactate concentrations against microneedle biosensor current downsampled to 1 min intervals (right). ISF, interstitial fluid.
Biosensor agreement with venous blood lactate
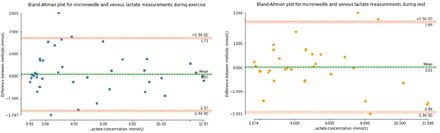
Venous blood lactate was used to calibrate biosensor current to compare agreement between measurements. We calibrated the biosensors separately depending on whether data was obtained from the exercise, or resting phase given observed patterns of microneedle current: venous lactate relationships (see online supplemental appendix). In general, comparison in both phases show good overall mean agreement with a 95% CI difference of ±1.89 mmol/L (figure 4).
Bland-Altman plots of agreement between biosensor and venous blood lactate aggregated for all five participants. Analyses were separated by phase of the study between exercise and rest. The left plot shows data obtained during exercise (0–30 min), and the right plot shows data during rest (30–60 min). The horizontal axes show the range of lactate observed in the study and vertical axes difference in agreement between measurements. The 95% CI (±1.96 of SD) is shown in orange horizontal lines.
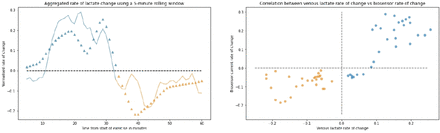
We analysed the performance of the biosensors to detect the change in lactate over time, given lactate clearance represented a clinically relevant endpoint. Biosensor and venous lactate data were normalised to the same unit and rates of change were derived by the mean gradient of a rolling-window average spanning 5 min (figure 5). The median response times of the microneedle patch estimated by differences in the peak measurements at the inflection point was 5.0 (IQR −4.0 to 11.0) min.
Normalised rate of change for all participants using a rolling window against time (left). The continuous line shows rate of change for the biosensor and triangles show venous lactate change at 1 min intervals. The blue plots represent exercise phase and orange represents rest phase. The mean lactate peak occurred after 32 min after start of exercise. Scatterplot showing venous lactate rate of change against biosensor current (right).
Participant acceptability
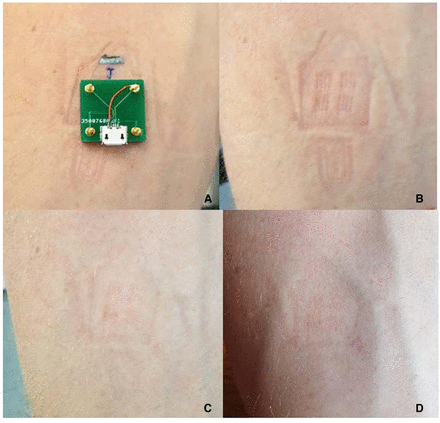
The mean score for discomfort at the patch site from the visual analogue score was 0.4/10, and degree of restriction rated 2.9/10 with comments relating to the wiring. All participants feel that the use of microneedle patch was preferable over frequent blood testing in a clinical context. A photograph of the skin at 0, 15, 60 min on removal of the biosensor after 120 min of placement for an individual participant is shown in figure 6 showing almost complete resolution of skin changes by 60 min of microneedle removal. No adverse events were reported during or after the study.
(A) Lactate biosensor in situ on participant forearm without connecting wires; (B) underlying skin after immediate removal of biosensor; (C) underlying skin after 15 min of biosensor removal; (D) underlying skin after 60 min of biosensor removal.
Discussion
This is a first-in-human pilot validating performance of a minimally invasive lactate microneedle biosensor in healthy volunteers. The biosensor provides a self-contained modality for measuring ISF lactate continuously and in real-time. We show that the microneedle biosensor placed on the forearm was able to detect lactate generated from leg exercise. Venous lactate ranges between 1.07 and 13.03 mmol/L were measured and biosensor current changed on average within 5 min of a change in venous lactate, showing correlation in terms of both levels and dynamics over time. Consistent results from microneedle biosensor signals were seen for all participants during the study period and the biosensor was well tolerated for the 2hour duration of use.
Lactate concentrations in pathological states serves as a sensitive, but non-specific biomarker. We used exercise as a proxy means to study transient increases in lactate. Although the physiological mechanisms for lactate production and clearance in exercise are different to that observed in pathology,3 our model is valid in capturing the end manifestation of hyperlactataemia. Increases in microneedle biosensor current closely followed venous lactate generation during exercise but the biosensor exhibited a slower decrease during rest. This has been described previously in exercise24 and may relate to individual physiological variability as well as a relative reduction in perfusion to skin tissue and ISF compartments postexercise, leading to delayed lactate clearance. Differences in physical activity and diet undertaken prior to the study could also contribute to individual differences and standardisation in future studies would be of benefit. In participants 4 and 5, we observed increases in microneedle current before that of venous lactate: possible explanations include biosensor and physiological variation, increased localised generation of lactate at the site placement and factors relating to the nature of microneedle placement within the skin.
Our microdialysis results support a relationship between ISF and venous lactate in exercise as well as microneedle current with ISF lactate concentrations. A more complete understanding of these relationships, as well as association between ISF lactate dynamics with clinical outcome will be a priority. In conditions such as sepsis, build-up of ISF lactate measured through microdialysis preceded changes in blood concentrations.15 The measurement of ISF lactate and/or its clearance dynamics could therefore provide clinically actionable information prior to the onset of hyperlactataemia which is regarded as gold-standard. However, these relationships are not consistently seen across clinical settings13 nor is it clear how states of impaired microvascular perfusion, such as that seen in falciparum malaria 25 affect ISF lactate dynamics.
In a previous study using the same microneedle platform, biosensors retained performance up to 24 hours of continuous use.20 These are attractive characteristics for use in a range of clinical conditions, particularly for low-income and middle-income settings where a robust device without moving parts supports its implementation. Future iterations of the biosensor could provide continuous ISF concentration readings through initial calibration against venous blood, or through a system of factory-calibration such as those found in continuous glucose monitoring systems.26 The ease of insertion and patient acceptability are also major advantages for their use in settings with limited healthcare resources, and furthermore supports a role in research, allowing for detailed interrogation of physiology in settings of shock particularly in children.27 The production of the device is scalable and the base cost is low,18 supporting implementation across a variety of settings. Expansion of sensing modalities using this platform is possible, allowing for multi-modal detection of relevant substrates, other biomarkers or therapeutic indices21—and will increase specificity of the tool. Linkage with decision-support systems and connectivity could provide benefits particularly in prehospital or ambulatory care settings.28 Carefully designed clinical studies will need to be carried out in order to investigate if continuous ISF lactate measurement ultimately translates into clinical benefit over intermittent blood measurements.
Limitations to our study include the small sample size and relatively short duration of biosensor use. Considerable sensor-to-sensor variation in terms of current output was also observed in light of small-scale fabrication processes and the pilot nature of the study. Ideally a longer study period would help understand the nature of biosensor performance over prolonged use. We observed an initial decrease of current from ±10 min from start of exercise which might be explained by the stabilisation phenomenon, whereby local physiological effects in response to biosensor insertion including changes to hydrogel and tissue perfusion may contribute. Differences in biosensor accuracy were seen in both low and high lactate concentrations in our study—optimisation of the biosensor dynamic range to ensure suitability for the intended clinical role will therefore be important. In clinical settings such as in shock, accuracy in the 2–8 mmol/L range might be of the most utility and the optimisation of biosensor membrane composition, thickness and deposition methods will have a role. We estimated the time-lag between the biosensor and venous blood comparing the time difference between peak lactate and peak current. It is likely that this relationship is more complex and not constant at all levels of venous lactate, nor between states of exercise and rest. However, the limitations of a relatively infrequent venous lactate sampling design as well as lack of additional calibration points meant we were unable to conduct these analyses in greater detail in this study.
Subsequent design and testing iterations will also need to address performance of the biosensor particularly in the low perfusion states observed in clinical settings: derangements in local acid–base balance resulting from shock or hypoxia could result in markedly different relationships for lactate between ISF and blood. Placement of the biosensor, and the composition of underlying subcutaneous tissue as well as depth of insertion could play a role in individual variability in our study.29 Standardised insertion methods onto the skin and methods of maintaining a consistent depth of penetration warrant investigation. Cross-reactivity with other compounds in ISF are known limitations for biosensors.30 Substrates such as uric acid, ascorbic acid and paracetamol undergo redox reactions at potentials similar to that used in lactate sensing, and can result in an increase in biosensor current. Although the Nafion membrane used has been shown to protect against these interferents31 and these compounds are not expected to change substantially during exercise, changes were observed on the control electrode (equivalent to the working sensor but without the enzyme). The implications of these changes are unclear and further work in clarifying the significance of these signals is ongoing within our group. Ensuring specificity in detection will be of importance for future use in clinical settings: improvements in biosensor designs, including the ability to reduce the potential applied at the electrode such as through use of direct electron transfer enzymes would offer significant benefits.
In conclusion, we demonstrate in a proof-of-concept study that the continuous measurement of ISF lactate using a minimally invasive microneedle biosensor is feasible, well tolerated and produces clinically actionable information in human participants. Work is ongoing to translate these findings for use in healthcare settings.
Data availability statement
Data are available upon reasonable request. Data is available upon request to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by London—Bloomsbury Research Ethics Committee (20/LO/0364) Participants gave informed consent to participate in the study before taking part. The study was sponsored by Imperial College London and conducted at the National Institute of Health Research/Wellcome Trust Imperial Clinical Research Facility (Imperial College London, UK). All researchers underwent Good Clinical Practice training and procedures conducted in accordance with the 1964 Declaration of Helsinki and later amendments.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Correction notice This article has been corrected since it was published Online First. Orcid Id of Saylee Jangam has been added.
Contributors DKM, DO'H and AHH conceived and designed the study. SJ, DO'H, SANG, DMEF, AEGC were responsible for the design, fabrication and all technical aspects of the microneedle biosensor. SANG and MGB were responsible for microdialysis. DKM, RW and AHH performed the clinical study. DKM performed the analyses and wrote the first draft of the manuscript. All authors contributed to the revision of the manuscript and have approved the final version to be published. DKM accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding The research was funded by the Department of Health and Social Care, Centre for Antimicrobial Optimisation, at Imperial College, London. This report is independent research funded by the Department of Health and Social Care. Infrastructure support was provided by the NIHR Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. AHH is a National Institute for Health Research (NIHR) Senior Investigator. DKM is supported by the Wellcome Trust (215010/Z/18/Z). DMEF is supported by the funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 825549 (ELSAH project).
Disclaimer The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the article. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the UK Department of Health.
Competing interests AEGC is the founder of a company ‘Continuous Diagnostics Ltd’ exploring applications of microneedle sensing technologies. All other authors have no competing interests to declare.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.