Article Text
Abstract
Early detection of physiological deterioration has been shown to improve patient outcomes. Due to recent improvements in technology, comprehensive outpatient vital signs monitoring is now possible. This is the first review to collate information on all wearable devices on the market for outpatient physiological monitoring.
A scoping review was undertaken. The monitors reviewed were limited to those that can function in the outpatient setting with minimal restrictions on the patient’s normal lifestyle, while measuring any or all of the vital signs: heart rate, ECG, oxygen saturation, respiration rate, blood pressure and temperature.
A total of 270 papers were included in the review. Thirty wearable monitors were examined: 6 patches, 3 clothing-based monitors, 4 chest straps, 2 upper arm bands and 15 wristbands. The monitoring of vital signs in the outpatient setting is a developing field with differing levels of evidence for each monitor. The most common clinical application was heart rate monitoring. Blood pressure and oxygen saturation measurements were the least common applications. There is a need for clinical validation studies in the outpatient setting to prove the potential of many of the monitors identified.
Research in this area is in its infancy. Future research should look at aggregating the results of validity and reliability and patient outcome studies for each monitor and between different devices. This would provide a more holistic overview of the potential for the clinical use of each device.
- vital signs
- remote monitoring
- outpatient
- wearables
Statistics from Altmetric.com
Introduction
Regular vital signs monitoring is a common inpatient care intervention, which aims to facilitate the early recognition of abnormal physiological parameters in deteriorating patients. Derangements in vital signs is a known predictor of cardiac and respiratory arrest,1 and early detection of deterioration has been shown to improve patient outcomes including mortality and quality of life.2 Traditional intermittent manual vital signs monitoring, such as early warning score systems, risks undetected patient deterioration through inadequate frequency of monitoring.3 Wearable remote monitoring technologies, aided by wireless data transmission, allow continuous monitoring of patients’ vital signs and introduce the possibility of physiological monitoring in the outpatient setting.
Until recently, outpatient vital signs monitoring has been mostly limited to ECG monitoring with Holter devices. These monitors have been used for over 40 years as a non-invasive method of continuously monitoring heart rate and ECG for set periods of time.4–6 Holter monitors do not allow real-time monitoring or monitoring of other vital signs such as oxygen saturation, blood pressure, respiratory rate and temperature.7 Recent improvements in battery technology and wireless data transmission alongside the advent of smartphones have heralded advances in wearable monitors. In the last 15 years, wearable monitors have been developed that incorporate multiple sensors, intelligent processing, alarms to support medical decisions and interactions with the health provider.
There are a number of wearable remote monitoring systems available. The level of evidence to support these systems is variable and the evaluation of remote wearable monitoring systems has been largely limited to the inpatient setting. As overall healthcare burden increases and scarcity of hospital beds leads to accelerated discharges, there is increasing interest in the application of remote monitoring in the outpatient setting.8
With increased interest in the use of remote outpatient monitoring, there is a clear need for a collective analysis of the efficacy of currently available wearable remote monitoring systems. At present, the only available review of remote monitoring systems applies to the inpatient setting and focuses solely on the accelerometer functions of wearable devices.9 This is the first review to compile the evidence for wearable devices for outpatient physiological monitoring. This study aims to provide a comprehensive overview of currently available systems and to evaluate and synthesise the evidence for each system to identify areas that require further evaluation.
Methods
Study design
The study has been conducted in the form of a scoping review to map all the existing literature on the topic to allow a broad overview of the area and identification of gaps in the evidence.
Inclusion criteria
Studies were selected according to the criteria outlined below:
Types of studies
Included studies reported evidence on outpatient wearable vital signs monitors. Peer-reviewed articles, trial registrations and grey literature such as white papers were included. As many of the wearable devices are in the early stages of evaluation, confining the search to peer-reviewed publications was not appropriate.
Types of participants
Studies were limited to those involving outpatients.
Types of interventions/comparators
The monitors under review were limited to those that could function in the outpatient setting with minimal restrictions to the patient’s normal lifestyle, while measuring any or all of the vital signs: heart rate, ECG, oxygen saturation, respiration rate, blood pressure and temperature. Only monitors that allow real-time monitoring were included.
Types of outcome measures
The selection of studies was not limited by the outcome measures reported. Evidence included reliability studies, evaluations of patient perspectives, clinical evaluations reporting patient outcomes and studies where the device was used in a trial environment but not tested in itself. Outcomes were collected as reported in the individual studies.
Exclusion criteria
Studies were excluded if they reported only the development of the technology for such monitors. Studies were excluded if they reviewed monitors that were no longer in production or those that were not wearable or functional only in the inpatient setting.
Information sources
Electronic searches
The search was conducted in two stages. MEDLINE, ClinicalTrials.gov, NICE.org, Google and PubMed Central (PMC) were searched for articles published from 1996 to June 2018 using the search terms outlined in box 1. Once the monitors were identified, a second search was undertaken; table 1 outlines the search terms and strategy used in the second search.
The initial search criteria
Vital Signs AND Outpatient Monitor* OR
Vital Signs AND Monitor* OR
Vital Signs AND Outpatient OR
Fitness AND Monitor
The search terms used in the second search
Searching other resources
References and citations of selected studies were searched to ensure completeness. Company websites for each of the products were also searched for white papers and links to peer-reviewed publications.
Data collection and analysis
Selection of studies
Studies were initially screened by title and abstract followed by a full text review. Studies for screening were recorded in a Microsoft Word document (Microsoft Word for Mac 2011, V.14.3.9, Microsoft, USA). Selected studies were collected and stored on Mendeley (V.1.19.2, Elsevier, USA).
Data extraction
Data were compiled in a table in Microsoft Word. This included information on the trade name, type of monitor, vital sign(s) measured and level of evidence.
Data synthesis
A narrative synthesis approach was used to summarise study findings. Monitors were grouped according to the type of device. Commonalities were sought in the type and volume of evidence substantiating each monitor. This evidence was then synthesised to assess the gaps in the literature for each monitor. Quantitative data synthesis was avoided due to the heterogeneity of outcome measures reported by selected studies.
Assessment of risk of bias in included studies
Due to the heterogeneity of the included study types and the preponderance of grey literature a formal assessment of risk of bias was not undertaken.
Results
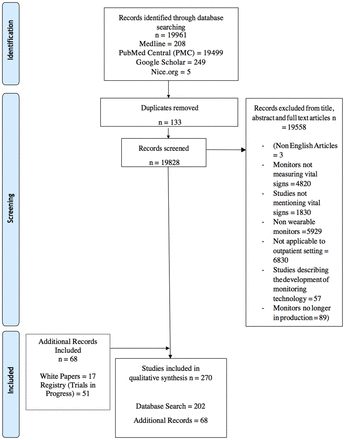
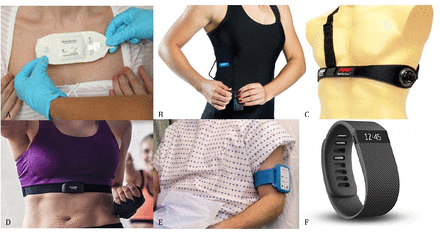
In total, 270 papers were identified that met the eligibility criteria. Figure 1 illustrates the selection process. Thirty wearable monitors were examined: 6 patches, 3 clothing-based monitors, 4 chest straps, 2 upper arm bands and 15 wristbands. Examples of each of these devices are illustrated in figure 2. The monitoring of vital signs in the outpatient setting is a developing field with differing levels of evidence for each monitor. The evidence base for each monitor is summarised in tables 2–7.
PRISMA diagram of search and eligibility process
Examples of outpatient monitoring devices included in this study. (A) Medical Grade Adhesive Patch: from Sensium, Abingdon, UK. (B) Clothing with embedded sensors: from Hexoskin, Montreal, Canada. (C) Chest strap: from Medtronic, Maryland, USA. (D) Chest strap: from Polar Electro, Warwick, UK. (E) Upper armband: from Current, Edinburgh, UK. (F) Wristband: from Fitbit, San Francisco, USA.
Summary table for all reviewed fitness wristband monitors
Summary table for all reviewed patch monitors
Summary table for all reviewed clothing monitors
Summary table for all reviewed chest strap monitors
Summary table for all reviewed upper arm band monitors
Summary table for all reviewed medical wristband monitors
Patch monitors
The review identified six wireless monitoring patches. All of the patch monitors measure heart rate and adhere to the patient with disposable stickers. Table 2 summarises the vital signs recorded by and the current evidence available for each device.
The patch with the most evidence was the SensiumVitals system, a patch monitoring heart rate, respiratory rate and temperature. Nine of its 17 total publications were case studies evaluating the use of the SensiumVitals system in a variety of clinical contexts from diagnosing meningitis10 to detecting opioid-induced respiratory depression. The authors conclude that the patch can feasibly be used in these contexts given its high levels of accuracy and patient compliance. However, it should be noted that these papers were written and published by Sensium Case Studies, a subgroup of the company producing the SensiumVitals patch.
Five of the six patches examined have validity or reliability studies. Of these, two have validity data but have not been tested in a clinical setting with no patient outcome studies. This may be due to the developing nature of the patch market with many patches in the early stages of product release.
For example, the patch with the least evidence was the Kenzen Patch with zero peer-reviewed publications and grey literature; it currently also lacks FDA certification. The product works differently from the other mentioned patches by analysing the biomarkers available in an individual’s sweat such as sodium and glucose in addition to measuring heart rate and temperature. Currently being marketed at athletes for its sweat-analysing biosensor, several American sports teams such as the San Francisco 49ers have signed up to trial this patch.
Clothing monitors
The three clothing-based monitors examined in this study function via monitors embedded in wearable garments. These items include a sports bra (OmBra), sports shirt (Hexoskin) and vest to be worn under clothing (Nuubo Wearable ECG). Table 3 summarises the vital signs recorded by and the current evidence available for each device.
The clothing monitor with the most evidence was Hexoskin with 42 relevant peer-reviewed publications. Hexoskin’s smart shirts are embedded with sensors and have a pocket for a Bluetooth recording device that enables data transmission to the wearer’s smartphone. The most common form of peer-reviewed publication for Hexoskin was a trial in which the monitor was used to measure vital signs, but was not evaluated in itself (22 studies). Of the vital sign monitoring functions of Hexoskin, heart rate monitoring was the most common used in these studies and oxygen saturation was the least common. The studies were wide ranging in population and setting, from badminton performance analysis11 to the physiology of a paraglider.12 The accuracy of Hexoskin was validated in the results of 18 studies using comparative apparatus including pneumotachographs13 and Metamax 3B14 for stationary and moving respiration rate. From these, it can be concluded that the vital signs monitoring functions of Hexoskin are accurate for use in the outpatient setting. These findings may be corroborated by current ongoing trials evaluating the use of Hexoskin in home-based cardiac patients.15
The clothing monitor with the least evidence was the OmSignal OmBra with zero relevant peer-reviewed publications and grey literature consisting of eight white papers produced by OmSignal. Of these, all involved validating OmSignal algorithms for analysing the two vital sign monitoring functions of OmBra, heart rate and respiratory rate.
Chest strap monitors
All four chest straps examined in this study involve devices that strap around the patient’s chest with embedded sensors to monitor vital signs. Table 4 summarises the vital signs recorded by and the current evidence available for each device.
Of the chest straps, Zephyr BioHarness 3 had the most relevant peer-reviewed publications. Transmitting data via Bluetooth, this chest strap uses sensors to detect respiratory rate, ECG, heart rate and temperature. Of 57 peer-reviewed publications, 47 described trials where the monitor was used but not tested. In these studies, the Bioharness 3 was used to visualise the physiology of various physical activities from basketball16 to cricket.17 The results of these studies suggest the Bioharness 3 can be used in ambulatory outpatient monitoring. Two studies found the Bioharness 3 to be equally18 or more sensitive19 than current hospital methods of respiratory rate detection.
The chest strap with the least evidence was QardioCore with zero literature due to its very recent product release in 2018.
Upper arm band monitors
Both upper armbands examined in this study strap around the patient’s upper arm and consist of cloth straps with a monitoring screen attached. Table 5 summarises the vital signs recorded by and the current evidence available for each device. Snap40 measures blood pressure; this is the least common vital sign measured by the monitors reviewed in this study. The other monitors in this review to measure blood pressure are Accurate 24, SpryHealth Loop, VisiMobile (see table 6) and Helo Lx (see table 7).
Both upper armbands had zero peer-reviewed publications. One white paper assessed the validity and reliability of Snap40 in comparison to a gold standard vital sign monitor as participants went about their daily lives for a week.20 The study found that Snap40’s measurements correlated with that of the gold standard monitor, demonstrating its ability to be used in real-world applications. Two further studies were conducted in hospital emergency departments.21 22 The first of these found that the use of Snap40 reduced time spent on vital sign collection by nurses from an estimated 12.33 hours to 1.2 hours per day.21 The study found positive links between less nursing time spent on vital sign collection and patient outcomes. The second study assessed 251 patients in a major UK emergency room for their patient perspective on the device.22 This included feedback on their level of comfort wearing the device and their confidence in its monitoring. The Snap40 white papers were funded by Snap40 Ltd.
Everion currently has no evidence in the public domain, but there is a single trial in progress. Conducted by a German university hospital, this is an outpatient validity and reliability study comparing the Everion’s ability to detect episodes of atrial fibrillation with that of the gold standard Holter monitor.23
Wristband: medical
Wristbands are by far the most common type of device currently available on the market. Attaching around the patient’s wrist, the wristbands studied have been divided into medical and fitness wristbands according to the marketing strategies of their respective companies. Medical wristbands have references to patients and how the devices contribute to health management on their websites. Table 6 summarises the vital signs recorded by and the current evidence available for each medical wristband.
The only medical wristband with relevant peer-reviewed publications was VisiMobile with one paper related to validity and reliability and two papers related to patient outcomes, although these were both conducted in the inpatient setting. This was also the wristband measuring the most vital signs: heart rate, ECG, blood pressure, respiratory rate and temperature. This wristband connects to sensors on the patient on body parts such as the thumb and chest. The first of the two patient outcome studies was conducted over a 3-month period in a wing of John Hopkins Hospital, USA, where 40% of sudden deaths were occurring.24 The study found that the introduction of continuous monitoring via Visimobile was associated with a reduction in failure-to-rescue events. The second of these studies surveyed the opinions of nurses on Visimobile after its use on their ward for four consecutive weeks.25 In comparison to products such as Hexoskin and Zephyr's Bioharness, Visimobile is unique in that all its clinical trials involve the evaluation of the monitor in a clinical context. This is fitting given its marketing specifically as a wristband for medical use. There is currently one ongoing trial examining the feasibility of its use in a general ward.26
Much like upper armbands, medical wristbands are a category very much in development. SpryHealth’s Loop was only recently deployed in 2018. Accurate24 is due for release in the summer of 2019. As a result, all three of the other medical wristbands examined have no evidence in the public domain.
Wristband: fitness
Table 7 summarises the vital signs recorded by and the current evidence available for each device. The fitness wristband with the most evidence was the FitBit with 18 relevant peer-reviewed publications. Of these, 12 studies were related to validity and reliability with a significant proportion of these concerned with the accuracy of the FitBit’s heart rate monitoring functions during various forms of exercise. The results of these studies suggest the FitBit provides accurate and reliable heart rate readings that can be used in the ambulatory outpatient setting. Current ongoing trials demonstrate the potential to expand the technology to the healthcare sector. These include a study where the monitor is incorporated into a mobile intervention for pulmonary arterial hypertension,27 and a study of patients with mood disorders using the heart rate function of the FitBit Charge series.28
The fitness wristbands with the least evidence were Helo Lx, Striiv Fusion Bio 2, Epson Pulse Sense and the Garmin Vivo Series. These had zero relevant peer-reviewed publications. Many papers were excluded as they failed to meet the inclusion criteria by focusing on the accelerometer and pedometer functions of these devices. Of these devices, only the Garmin Vivo Series devices had trials in progress, examining the patient perspective of the Garmin Vivo Series devices by measuring adherence and wear time.29 30
Discussion
This review aimed to quantify the evidence available for in-use and upcoming devices for wearable outpatient vital signs monitoring. By identifying the current gaps in the literature and the monitoring functions of these devices, this paper aims to assist clinicians and researchers to select appropriate remote monitoring devices based on existing evidence.
It is apparent that the monitoring of vital signs in the outpatient setting is a developing field, with evidence per monitor ranging from many relevant peer-reviewed publications to zero evidence in the public domain. The FitBit had the most trials in progress, expanding its influence in the field. Gaps in the literature exist with no FitBit patient perspective papers quantifying feasibility and adherence; however, 11 of the 2231–41 trials in progress will address this with their published results. These will report on compliance, feasibility and adherence from a variety of study populations.31–34
Gaps in the literature also exist for upper armbands and medical wristbands. These two types of devices had the fewest relevant peer-reviewed publications. Further research into the use of these wearable outpatient vital signs monitors should look at primarily evaluating their validity and reliability, patient perspectives and patient outcomes when using these devices, in order to validate the products for use in a clinical environment. Gaps in wearable monitor function exist for blood pressure monitors with only five monitors measuring blood pressure. This was followed by O2 saturations with only seven monitors assessing this.
Although there are a multitude of wearable monitors available to the consumer market, there is little evidence of commitment to the routine use of such devices in the healthcare setting. The award of a CE (Conformité Européenne) mark indicates conformity with health, safety and environmental protection standards for products sold within the European Economic Area, but does not guarantee the effectiveness of the product. The level of evidence required to support an application to a national decision-making body such as the National Institute for Health and Care Excellence (NICE) in the UK usually includes clinical trials and health economic analyses. These studies necessitate the commitment of funds, resources and time, thus impacting on the short-term profit margin of small companies. One potential solution to this problem is the partnering of commercial companies with academic institutions to provide high-quality, impactful evidence.
Strengths and limitations
The main strength of this review is that it is one of the first to give an overview of existing literature on wearable outpatient monitoring devices. Previous research into the field of wearable monitors can be classified into four broad categories: reviews of cardiac functions of monitors;42–45 reviews of the activity tracking function such as pedometers and accelerometers of monitors, particularly pertaining to wristband fitness trackers;46–49 reviews of respiratory monitoring functions;50 and miscellaneous reviews such as those evaluating the use of wearable monitors for monitoring stress,51 sleep52 and their use in clinical trials.53 None of these reviews of wearable devices were exclusive to general outpatient monitoring and the monitoring of vital signs. Thus, this review is one of the first of its kind to review wearable outpatient vital signs monitors.
Another strength of this review lies in its scoping nature. Given the developing field, a scoping review allows for quantification of all the evidence relevant to the research question available for each device. The inclusion of a broad range of literature ranging from grey literature such as white papers to registries of trials in progress to peer-reviewed papers allows for a more holistic picture of the evidence. This enables identification of the gaps in the evidence and the product market for wearable outpatient vital signs monitors.
The limitations of this review are the inclusion of only English language publications. Two publications in foreign languages were found,54 55 but they were not included due to the language barrier they posed. Furthermore, the quality of each study was not evaluated due to the huge variation in the types of evidence, the study methodologies and the outcomes assessed. An assessment of risk of bias was inappropriate given the scoping nature of the review and the preponderance of grey literature in the search results.
Future research
Future research should look at aggregating the results of validity and reliability and patient outcome studies for each monitor and between different devices. This would provide a more holistic overview of the potential for clinical use of each device as an outpatient vital signs monitor.
References
Footnotes
Twitter @MissCDowney
Presented at The findings of this review have been presented as a poster at the National Student Association for Medical Research (NSAMR) Conference in January 2019, and at the Association of Surgeons in Training International Surgical Conference in March 2019. The abstract will be published in the British Journal of Surgery.
Contributors CD and SS were involved in the conception of the work and designed the study. SS undertook the data collection and performed the analysis and interpretation. CD, HS and SS drafted the article. All authors (SS, CD, HS and DGJ) were involved in critical revision of the article and have given final approval of the version to be submitted.
Funding CD is in possession of a Doctoral Research Fellowship (DRF-2016-09-037) supported by the National Institute for Health Research. DGJ is funded by Bowel Cancer UK and RCS England. The research is supported by the NIHR infrastructure at Leeds.
Disclaimer The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, Health Education England or the Department of Health.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.