Article Text
Abstract
Objective The female condom is a non-hormonal barrier method that can protect from unintended pregnancy and sexually transmitted infections such as HIV. Female condoms are an important contribution to women’s reproductive health globally as they are the only woman-initiated method currently available that can provide dual protection. This article describes how human-centred design (HCD) was applied to the development of the Woman’s Condom—a second-generation female condom.
Methods A multidisciplinary team pioneered the application of HCD principles to develop a novel reproductive health product. The Woman’s Condom design incorporated feedback from both female and male users from multiple sites in the USA and Cuernavaca, Mexico; Durban, South Africa and Khon Kaen, Thailand to inform product development.
Results We developed and tested more than 50 design iterations reflecting various solutions to user-related concerns. The final locked design confirmed that the Woman’s Condom was easy to use, stable, comfortable and provided satisfactory sensation during sex for both partners. The ‘dissolving capsule’ to facilitate insertion and ‘soft cling’ design are key innovative features of the Woman’s Condom.
Conclusion The Woman’s Condom is a second-generation female (or internal) condom product that has been shown to be highly acceptable to users throughout the world. The Woman’s Condom’s special design features enable easy insertion, secure fit during use, good sensation and easy removal. Engaging users as codesigners through an HCD approach resulted in a female condom that meets the needs of women and men from diverse regions.
- women's health
- sexual health
- sexually transmitted diseases
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
Summary box
What are the new findings?
Employing a human-centred design process, we developed an innovative female condom to address consumers’ desire for easy to use, acceptable protection that is pleasurable for both partners.
Engaging users from multiple countries as codesigners strengthened design decisions resulting in a female condom that meets the needs of women and men from diverse regions.
Developing a product that is acceptable and meets user needs is not sufficient for market uptake of a consumer product; demand generation also is required.
Although we designed to be price competitive for low-income and middle-income country markets, external changes (ie, price reductions in the reference product) resulted in this product not being competitive for public sector tenders. Markets where consumers are willing to pay for higher product satisfaction could help raise awareness and build market demand.
How might it impact on healthcare in the future?
Even when developing a sensitive product like a female condom, incorporating input from users is possible and can help build acceptability into the product at each stage of design to meet the needs of intended audiences.
Introduction
Human-centred design (HCD) is generally viewed as an approach that brings the perspectives and ‘lived reality’ of the people the design is intended for into the product development process. Although the HCD approach is well established in the business sector, it has only been integrated into the global health arena relatively recently. The recent efforts of the global health community to incorporate HCD have been due, in part, to the intransigence of long-standing problems related to the introduction and use of novel health technologies and the need to apply fresh approaches to address them.1 Global stakeholders such as UNICEF and US Agency for International Development (USAID) encourage the use of HCD and have demonstrated its relevance for global health.2 3 HCD has also been proposed as a way to achieve global health equity4 and user-friendly tools have been developed to implement HCD in global health contexts.5
Recent reports of the application of HCD in global health feature predominately digital decision-making tools6–8 and systems design.9 10 Reports of using an HCD process for the development of medical devices are less common. The HCD process was used to design neonatal incubator technologies and supporting equipment to meet the needs of infant patients, family members and medical personnel,11 and a phototherapy device for treatment of neonatal jaundice.12 HCD thinking was used to ideate solutions for the prevention of lower urinary tract symptoms in women in the USA.13
HCD encompasses ideation, testing, learning and refinement based on the feedback from a sample of the intended audience. While human factors evaluations are part of product design and essential from a regulatory perspective, incorporating iterative user feedback can be challenging to obtain when the medical devices being developed are related to sexual behaviour. The sensitive and confidential nature of sexual behaviour has often precluded product innovators from actively incorporating HCD principles into the development of novel contraceptive technologies. Recently, HCD thinking was applied to develop strategies to increase the uptake and use of a new vaginal ring that includes Dapivirine, an antiretroviral drug for long-acting HIV14 prevention.
More than twenty years ago, PATH pioneered integration of HCD into contraceptive technology development. Using an HCD approach that incorporated users as codesigners throughout product development and testing, we developed an innovative female condom with features inspired by user input that provides an optimal use experience for both women and men. This article describes how HCD was applied to the development of the Woman’s Condom—a second-generation female condom.
In many countries, women experience significant morbidity and mortality due to unintended pregnancy, HIV and sexually transmitted infections (STIs). Currently, condoms are the only contraceptive method that also protects from HIV and STIs. However, gender inequality and cultural issues make it difficult for women to negotiate use of male condoms. Female condoms offer a woman-initiated option that protects from pregnancy and HIV/STIs. Creating a female condom that offers good sensation and is acceptable and attractive to both partners could reduce barriers women experience when negotiating condom use for safer sex.
In 2018, the US Food and Drug Administration (USFDA) down classified single-use female condoms from class III to class II—similar to male condoms—and changed the product description to ‘internal condom’ to degender the product category.15 Female condoms are an important contribution to women’s reproductive health globally as they are the only woman-initiated method currently available that can provide dual protection.
Methods
The design portion of this product development project was implemented over a 5-year period from 1998 to 2003. This was followed by clinical testing to generate evidence of safety and performance required for regulatory approvals. The initial need for a refined woman-initiated dual-protection product emanated from the voices of reproductive health advocates who attended the 1994 International Conference on Population and Development (ICPD) where they called for universal access to a full range of family planning methods to meet their needs including protection against STIs.16 At the request of USAID, PATH responded to this call by exploring core areas for improvement in the female condom product category and embarking on an HCD process to develop a redesigned female condom.
The Woman’s Condom was designed through a user-centred process to address consumers’ desire for a product that provides protection and is simultaneously acceptable, easy to use and pleasurable for both partners. The multidisciplinary design team consisted of product development engineers, designers, global health system experts, commercialisation advisors and research investigators. Women and their partners and family planning providers from Mexico, South Africa, Thailand and the USA were active participants throughout the period of product development. We opted to employ a user-driven design process to ensure product acceptability and sustained use. The learning we gained from our iterative and interactive design cycles (ie, design-build-test) allowed the design to evolve over time in response to better understanding user needs.
Through a literature review, assessment of currently available female condom products and formative research with women’s groups, healthcare providers, and sexual and reproductive health (SRH) advocates, we identified performance objectives required for a new female condom design that could address consumer needs for acceptability and ease of use. The performance objectives (box 1) provided the roadmap for developing a product that addressed challenges with existing products and set the benchmark against which prototype designs were evaluated by users in an iterative HCD process. We later developed product requirement specifications (PRS) to define evaluation indicators for functional performance and acceptability. The PRS criteria reflected a combination of existing product standards and PATH’s best understanding of user needs as embodied in the original design objectives. Throughout this design process, the Woman’s Condom product was developed according to national and international guidance documents for medical devices and vaginal products.17–19
Woman’s Condom performance objectives
Easy to handle and insert.
Easy to use (especially for new users).
Feels good during sex.
Comfortable for both partners.
Does not slip or move during use.
Easy to remove.
Aesthetically pleasing to end user.
Pricing similar to existing products.
We prioritised working with research sites with female researchers, who were committed to ICPD principals, and trusted sources for information and services among women in their communities. Our team developed a study protocol to systematically collect data from women of reproductive age (18–45 years) and their partners from multiple sites in the USA and Cuernavaca, Mexico; Durban, South Africa and Khon Kaen, Thailand to inform product development. We specifically chose to work with women from general populations who did not consider themselves sex workers to help normalise the use of female condom products. This was in contrast to previous female condom studies that focused on sex workers and women at high risk of HIV, which inadvertently stigmatised use of female condoms among some audiences.
We interacted with participants first via direct observation of clinical fittings, and later via open-ended questionnaires completed by the couple after each product use and in-depth interviews with female and male partners separately to assess the acceptability of the various prototype designs. Areas of inquiry related to performance requirements noted in box 1. We designed and evaluated features to allow the female condom to be inserted deeply into the vagina and remain stable during use; to have good sensation almost the same as ‘naked sex’; and to be small in profile, easy to insert and attractive. In parallel, we evaluated product materials and manufacturing processes to ensure the design would be manufacturable and had the potential to reach a unit cost that could be competitive and meet market requirements for this type of product given that female condoms are purchased primarily by international procurement agencies and governments for use in family planning/HIV programmes in low-income and middle-income countries (LMICs).
Our iterative design process started with bench testing prototypes for functionality and then proceeded to user testing. Initial user testing included in-hand evaluations of prototype designs, then fittings in a clinic setting and ultimately evaluations by women and their partners using the product during sex. For each round of evaluation, up to 20 women of varying body mass index, parity, age, and socioeconomic status per site participated. Overall, more than 200 women and their partners across the multiple sites evaluated Woman’s Condom prototypes and provided feedback to inform the design.
Very few women had prior experience with female condoms before these user assessments. During each of the successive rounds of evaluation, we recruited some new participants to join continuing participants from the previous round of evaluation. This provided continuity across evaluations to ensure that design modifications addressed the problems/issues that had been identified in the previous round and also provided feedback from naïve users.
Results
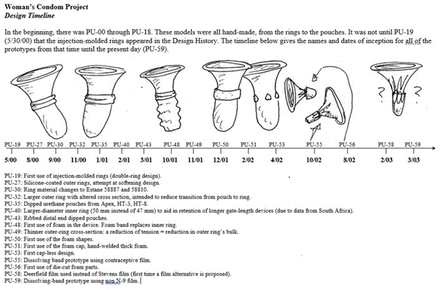
Figure 1 depicts the design history timeline for the Woman’s Condom beginning with an initial double-ring design that used injection-moulded rings as a retention feature and evolved eventually into four foam shapes for retention. The prototypes evolved from having a foam cap at the distal end of the pouch to loading the pouch into a dissolving cap which reduced product size and aided insertion.
Design history timeline for the Woman’s Condom polyurethane (PU) prototypes from May 2000 to March 2003.
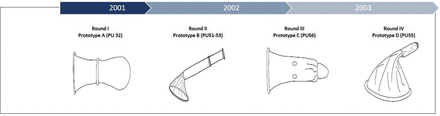
Overall, we developed and tested more than 50 designs reflecting various solutions to user-related concerns such as ease of handling and stability during use (figure 2). At each step along the way, we collected feedback to refine the product features and characteristics that would affect ease of use and acceptability. Our goal was to build acceptability into the product at each step along the way by exploring product attributes such as insertion aids and retention methods and understanding the tradeoffs between design features, functionality, acceptability, manufacturability and cost. Selected design modifications based on user feedback over the course of the Woman’s Condom design history are shown in table 1.
Design evolution based on user evaluations. PU, polyurethane.
Design evolution based on user evaluations
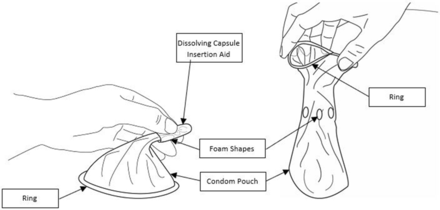
The final design that was evaluated in the validation study, PU63 (2003), reflected final refinements based on round IV, prototype D. The dissolving band evolved into the dissolving capsule insertion aid mentioned below. Figure 3 depicts the final design and specific feature set of the Woman’s Condom. The Woman’s Condom is a thin plastic pouch that is inserted in the vagina prior to intercourse, and stays in the vagina during intercourse, providing a physical barrier between partners' genitalia. Assembly is completely by thermo welding; no adhesives are used. Key design features are:
Very thin film pouch (1.2 mil urethane film) provides a high level of sensation and comfort.
Low profile, soft urethane ring at the open end of the pouch gently hugs the body, providing comfortable, flexible coverage.
Four foam shapes (small, thin sections of hydrophilic urethane foam) cling lightly to vaginal walls, ensuring stability during use.
Dissolving capsule insertion aid (made of polyvinyl alcohol (PVA) used in contraceptive film) contains condom pouch for easy handling during insertion and a rounded tip for comfort.
The woman’s condom is unlubricated and designed for single use. Water-based lubricant is provided in the package.
Final design and feature set of the Woman’s Condom.
After four rounds of iterative development, user feedback indicated the prototype design met the performance objectives, so the design was ‘locked’. Then we implemented a design validation study among 60 couples from three countries to assess performance and acceptability of the Woman’s Condom. Couples were asked to use the prototype device during sex three times and results were compared with the PRS.
Results from the validation study using the locked design among users in Mexico, South Africa and Thailand confirmed that the Woman’s Condom was easy to use, stable, comfortable and provided satisfactory sensation during sex for both partners.20 Further, a phase I randomised cross-over clinical study21 at three sites in the USA confirmed the performance, safety and acceptability of the Woman’s Condom compared with the currently available female condom product (FC1) among 75 couples. Results from a study22 in South Africa that compared three female condoms in development with the one female condom product that was approved by the USFDA found that Woman’s Condom performed as well or better than other female condom products.
In general, results from these studies showed that the Woman’s Condom performed well when compared with other female condom products. Women who reported preferring the Woman’s Condom cited ease of use, stability and comfort as their main reasons. In the USA, a multicentre open-label phase III trial23 of the woman’s condom was conducted to evaluate the safety, contraceptive effectiveness and acceptability24 of the Woman’s Condom over 6 months (183 days) and ≥6 menstrual cycles. Results from this study confirmed the overall acceptability of a new female-initiated barrier contraceptive option that can protect against both pregnancy and STIs. These clinical studies confirmed the design was well-accepted and performed as well, if not better, than other female condoms.
In 2008, PATH licensed the Woman’s Condom design for manufacturing and commercialisation to the Dahua Medical Apparatus of Shanghai (DAHUA), a Chinese medical device company with expertise in manufacturing thin film polymers (https://shdahua.en.ec21.com/Female_Condom--5904684_5904705.html). PATH provided technical assistance for pilot production, production scale-up and regulatory applications. DAHUA designed and developed unique manufacturing equipment that consolidated and automated several manufacturing steps. This new semiautomated production line allowed DAHUA to produce millions of Woman’s Condoms annually while maintaining the quality required by international standards. In 2010, the European CE mark was awarded to the Woman’s Condom manufactured by DAHUA, which certified the safety and performance of this medical device according to the intended use. Regulatory approvals were obtained in China (2011), South Africa (2013), Malawi and Zambia (2014) and Brazil (2016).
By 2015, DAHUA built, installed and validated the new semi-automated production line with a capacity to manufacture nearly 2.5 million Woman’s Condoms annually. In 2016, the Woman’s Condom manufactured by DAHUA achieved qualification under the United Nations Population Fund/World Health Organization (UNFPA/WHO) Prequalification Programme for Female Condoms. This internationally recognised certification ensures the quality of products procured by international agencies such as the United Nations and is often a tender requirement of country governments.
Around the same time, we conducted a cost-effectiveness study to estimate the potential dual health impact and cost-effectiveness of a Woman’s Condom distribution programme in 13 sub-Saharan African countries with HIV prevalence rates >4% among adults aged 15–49 years. Results from this cost analysis showed that the Woman’s Condom was very cost-effective in all 13 countries; for every 100 000 used, about 194 unintended pregnancies and 24 HIV infections would be averted.25
Discussion
The International Standards Organisation26 has identified HCD as a complex practice characterised by six principles:
The design is based on an explicit understanding of users, tasks and environments.
Users are involved throughout design and development.
The design is driven and refined by user-centred evaluation.
The process is iterative.
The design addresses the whole user experience, including the context in which the user finds his/herself.
The design team includes multidisciplinary skills and perspectives.
Our development of a refined female condom product followed these six principles of HCD. Notably, our understanding of the SRH needs of couples and especially women in LMICs is based on decades of experience creating appropriate technology solutions. We used an iterative process that included both female and male partners from diverse settings throughout the design process. The design was informed and refined by feedback obtained during user-centred prototype evaluation, where the product was used during sex over multiple sex acts. We systematically interacted with users throughout the entire user experience beginning with exploring how, where and when they have sex, talk to their prospective partners about the possibility of using something to protect against unintended pregnancy and STIs, use a female condom successfully and pleasurably, and dispose of the product properly. We were able to address these aspects of a multi-faceted sexual experience because our multidisciplinary team brought various lenses to this work including engineering, social science, public health and business, and we worked with research teams who were trusted by women in their communities.
Challenges and lessons learnt
Proven acceptability of a product, however, does not always translate into uptake. Investment in demand generation is critical with any new product entering the market. In the case of the Woman’s Condom, a lack of coordination between supply and demand limited market penetration.27 Demand generation efforts focused on LMIC markets primarily in sub-Saharan Africa and China (because the manufacturer was based there and wanted to build the domestic market). Early market introduction studies28–30 in both of these countries as well as Zambia31 confirmed that consumers liked the product.
However, most female condom products are purchased by international procurement for distribution in LMICs. The global marketplace is highly price sensitive and the lowest cost products are the most competitive, particularly for these types of international tenders. To address this, we delineated a performance specification related to creating a product that was priced similarly to currently available female condom products. When the design effort first began, the market leader was being purchased by international donors at a price of around US$0.55 each. As the Woman’s Condom product moved closer to market entry, the price of the reference condom when purchased by international donors lowered dramatically from US $0.55 to about US$0.35 each at high production volumes due to a change in product material from polyurethane to nitrile and shift to lower cost production facility, which allowed for a lower cost manufacturing process. At the same time, the price of the Woman’s Condom (at a production volume of 2.5 million) was around US$0.80 each. The current design and related manufacturing process of the Woman’s Condom precludes ever reaching this lower price point.
Throughout product development, PATH worked with manufacturers and consultants familiar with female condom manufacturing to inform and check design decisions with an eye toward achieving a design that could be manufactured at a competitive price. The final design included the lowest cost features and components that met design objectives with high levels of user satisfaction. The Woman’s Condom is made of a very thin polyurethane film, which provides the good consumer experience in terms of sensation and satisfaction that women and men asked for, but resulted in a product that is not competitive for public sector tenders (especially at initial production volumes). The welding process used to form the Woman’s Condom pouch using thin film is technically challenging and the polyurethane material is expensive compared with female condoms made from latex and synthetic nitrile latex. The Woman’s Condom manufacturing process requires several steps, which increases overall labour and associated costs. One design fix that has been suggested is to remove the foam shapes to reduce product cost. A study in South Africa found the modified Woman’s Condom design without foam shapes performed as well as the original Woman’s Condom design.32
A relatively more expensive female condom product may fare well in developed and middle-income countries where consumers are willing to pay for a product that provides greater satisfaction. In the United States, a regulatory application for the woman’s condom has not yet been submitted. The Contraceptive Clinical Trials Network of the National Institutes of Health and Child Development implemented a phase III contraceptive effectiveness trial of the Woman’s Condom.23 Acceptability results have been published and effectiveness results are expected within the next year. Favourable results from this clinical trial showing that the Woman’s Condom has good effectiveness may incentivise a company to introduce the product as an aspirational product where consumers are able and willing to pay for a heightened consumer experience.
Conclusion
The Woman’s Condom is a second-generation female (or internal) condom product that has been shown to be highly acceptable to users throughout the world. The special design features of the Woman’s Condom enable easy insertion, secure fit during use, good sensation and easy removal. Engaging users as codesigners through an HCD approach resulted in a female condom that meets the needs of women and men from diverse regions.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Ethics approval
We received ethics approval by the PATH Research Ethics Committee and ethics review committees at each of these sites for these non-randomised, non-significant risk studies among couples in a monogamous relationship who were not at risk of pregnancy and at low risk of sexually transmitted disease in the countries where the activities occurred.
Acknowledgments
We would like to acknowledge the effort and commitment of the PATH Woman’s Condom Product Development Team led by Glenn Austin; our research partners, Dilys Walker (formerly) of the National Institute of Public Health in Cuernavaca, Mexico; Mags Beksinska of MRU (MatCH Research Unit) formerly the Reproductive Health Research Unit, University of Witwatersrand in Durban, South Africa; and Earmporn Thongkrajai of the Department of Community Nursing, Khon Kaen University in Khon, Kaen, Thailand; CONRAD/Eastern Virginia Medical School and their associated research sites; and the women and men who willingly shared their experience and ideas to create the Woman’s Condom.
References
Footnotes
Contributors Both authors were involved substantively in all aspects of the product development work including the conception and design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Both authors have provided final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding PATH developed the Woman’s Condom with support from the following donors and agencies: CONRAD, Eastern Virginia Medical School, under USAID Cooperative Agreement #HRN-A-00-98-00020-00; the United States Agency for International Development (USAID) under the terms of the HealthTech IV Cooperative Agreement #GPH-A-00-01-00005-00; Netherlands Ministry of Foreign Affairs; Bill and Melinda Gates Foundation; I+Solutions/Universal Access to Female Condoms; and other donors. Support for the preparation of this manuscript came from KESSEL Medintim, GmbH (Waldorf, Germany).
Disclaimer The content of paper is solely the responsibility of the authors and does not necessarily represent the official views of the donors.
Competing interests Both authors work for PATH, a global non-profit that improves health (www.path.org) and were actively involved in the product design and market development efforts around the Woman’s Condom. These efforts were supported by various donors including the United States Agency for International Development (USAID), Netherlands Ministry of Foreign Affairs, and Bill & Melinda Gates Foundation.
Provenance and peer review Not commissioned; externally peer reviewed.