Article Text
Abstract
Objective This study aimed to assess a non-invasive haemoglobin (Hb) sensor NBM 200 in pregnant women in a rural Indian setting.
Methods The study population consisted of women between 3 and 5 months of pregnancy, from 33 villages in Tuljapur and Lohara blocks of Osmanabad district, Maharashtra, between April 2014 and June 2015. Hb measurements obtained from the non-invasive sensor NBM 200 were compared with measurements obtained from an automated haematology analyser Sysmex XP-100, using the Bland Altman method and Spearman's Rank correlation coefficient. Interclass correlation coefficient (ICC), sensitivity and specificity values were used to assess the anaemia diagnostic accuracy of NBM 200 against the gold standard (Sysmex XP-100).
Results Data were obtained from 269 pregnant women (median age: 21 years, IQR: 19–23 years). Hb levels estimated by the Sysmex XP-100 analyser ranged from 5.5 g/dL to 14.1 g/dL (mean: 10.0 g/dL, SD: 1.28), while measurements obtained from NBM 200 ranged from 9.5 g/dL to 14.6 g/dL (mean: 11.9 g/dL, SD: 1.43). Spearman's test found a significant, moderately positive correlation between the two methods (rs=0.4, p<0.001), ICC was 0.22, and the Bland-Altman analysis showed a mean difference of −1.8 g/dL (95% CI −2.06 to −1.71), indicating a systematic overestimation of Hb using the NBM 200. The NBM 200 showed low sensitivity (33.7%; 95% CI 27.3% to 40.5%) but high specificity (91.8%; 95% CI 81.9% to 97.3%) for the diagnosis of anaemia.
Conclusions Hb measurements obtained from the NBM 200 were higher with consequent underestimation of anaemia as compared with the gold standard reference method. This limits the use of the NBM 200 as an anaemia diagnostic test in our study population consisting of women during pregnancy.
- Anaemia
- Non-invasive Haemoglobin
- Pregnancy
- NBM 200
- India
Statistics from Altmetric.com
Introduction
Haemoglobin (Hb) is commonly used to measure anaemia status using an automated haematology analyser,1 but this requires both blood withdrawal and access to laboratory facilities. In developing countries, many individuals, often those with greater health needs, do not have easy access to diagnostic facilities.2 Recently, the non-invasive Hb sensor NBM 200 was introduced for Hb assessment, which provided an opportunity for anaemia screening at the population level in developing countries.1 ,2 The NBM 200 sensor is a non-invasive portable device consisting of a finger probe and a processing unit with digital display. The finger-blood analyser uses occlusion spectrometry to estimate Hb in approximately 60–100 s.3
Recent studies conducted to validate NBM 200 mainly involved blood donation centres within hospital settings.4 There is a strong clinical need to measure Hb levels accurately in settings where laboratory access is not easy, particularly during pregnancy where repeated measurements are required. However, there are no published evidences on the application of non-invasive Hb sensors outside hospital settings for antenatal anaemia screening.2 ,4 Thus, it is an important area of research considering the high prevalence of anaemia in women during pregnancy in a country like India.5 ,6 Moreover, there is little evidence for the accuracy of non-invasive Hb sensors used in rural community settings. Therefore, the present study aimed to assess non-invasive Hb measurements (using the NBM 200 sensor) against measurements obtained from a gold standard method using an automated haematology analyser (Sysmex XP-100)7 among pregnant women living in rural India.
Materials and methods
A cross-sectional study was conducted in 33 villages from Tuljapur and Lohara blocks, Osmanabad district, in the Indian state of Maharashtra with a catchment population of approximately 64 000 individuals. The primary aim of the study was to investigate the prevalence and risk factors of anaemia in pregnant women; this paper reports on a secondary aim which was to validate the use of the non-invasive Hb sensor NBM 200 as a screening test for anaemia in this population. The study population consisted of pregnant women between 3 and 5 months of pregnancy who provided data for the period 24 April 2014 to 30 June 2015. Each participant was recruited after obtaining written consent in the presence of a witness and the primary investigator (ASA). Trained data assistants obtained the non-invasive Hb measurements in accordance with the manufacturer's guidelines for NBM 200 (Orsense Ltd, Nes-Ziona, Israel). Exclusion criteria were physical deformity of the thumb of the non-dominant hand, any injury/ulcerations, localised infection, oedema and skin breaks.8 The gold standard measurement was obtained using a fully automated haematology analyser (Sysmex XP-100, Japan) on a venous blood sample at the haematology laboratory based in the Halo Medical Foundation's (HMF) registered hospital at Andur, Maharashtra.7 The non-invasive test was conducted in a sitting position in the field (either at the participant's home or in the village health/nutrition centre) followed by blood withdrawal within a 5 min interval.9 For every participant, the non-dominant hand was used for venous blood withdrawal from the median cubital vein and the thumb of the same hand was used to obtain the non-invasive measurement.9 The venous blood was obtained under an aseptic protocol using a 2 mL disposable sterile syringe. Each participant was asked to wait for 10 min after completion of both tests to ensure no adverse effect occurred post blood withdrawal. The blood sample was transferred to a 2 mL vacuum tube containing EDTA anticoagulant, which was then safely transported in a standard blood carrier container and tested in the HMF laboratory using Sysmex XP-100 within 4 h of withdrawal. The analyser was calibrated everyday according to the manufacturer's guidelines and standard laboratory protocol.7
Hb measurements obtained from each method were used to categorise each participant as having anaemia (Hb <11 g/dL) or not.10 We also classified participants as having severe anaemia if their Hb values were less than 7.0 g/dL.10 Spearman's rank correlation coefficient (rs) and interclass correlation coefficient (ICC) were used to assess correlation and accuracy, respectively, between the Hb measurements obtained from the two methods. Agreement between the two methods was further investigated using the Bland-Altman analysis.11 The diagnostic accuracy of NBM 200 was then assessed against Sysmex XP-100 (the gold standard comparison in our study).7 The sensitivity (the proportion of true positives correctly identified by NBM 200), specificity (the proportion of true negatives correctly predicted by NBM 200), positive predictive value (PPV), that is, the proportion of NBM 200 anaemia positive participants for whom the anaemia diagnosis was validated by Sysmex XP-100, and negative predictive value (NPV), that is, the proportion of NBM 200 anaemia negative participants who were non-anaemic as per Sysmex XP-100, were estimated. All statistical analyses were conducted using Stata Statistical Software (V.13.1, Texas, USA). The study was approved by the Institutional Ethics Committee of Government Medical College of Aurangabad, India (Reference number: Pharma/IEC/GMA/196/2014), and also by the Nottingham University Medical School Research Ethics Committee (Reference number: E10102013).
Results
We approached 278 eligible pregnant women from 33 villages of Osmanabad district, of whom 271 (97.5%) agreed to participate in the study. Owing to a technical sensor error, the non-invasive Hb readings of two participants could not be obtained, leaving 269 samples (96.8% of the eligible women) for analysis.
The median age of study participants was 21 years (IQR: 19–23 years). The Hb estimated by Sysmex XP-100 (gold standard) ranged from 5.5 g/dL to 14.1 g/dL (mean: 10.0 g/dL, SD: 1.28), while the Hb estimated by NBM 200 ranged from 9.5 g/dL to 14.6 g/dL (mean: 11.9 g/dL, SD: 1.43). The Sysmex XP-100 analyser (gold standard) results showed a high prevalence of anaemia (77.3%) in the study population with 5 (1.9%) individuals classified with severe anaemia (table 1). The NBM 200 sensor measurements classified 27.9% of the study population with anaemia and none with severe anaemia.
Cases of severe anaemia which were measured as moderate anaemia by the non-invasive sensor
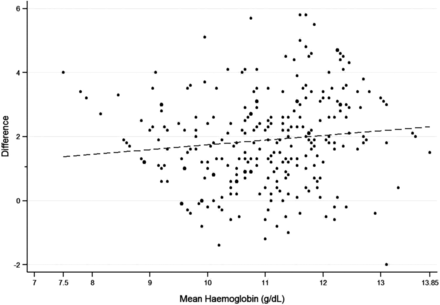
The Bland-Altman plot is presented in figure 1. A comparison of measurements from the two Hb estimation methods yielded a mean difference of −1.8 g/dL (95% CI −2.06 to −1.71), suggesting a systematic overestimation of Hb using NBM 200. In 11.2% cases, NBM 200 yielded Hb measurements that were equal to or marginally higher than the measurements from Sysmex XP-100. A statistically significant, moderately positive correlation was observed between the NBM 200 and Sysmex XP-100 measurements (Spearman's correlation coefficient rs=0.4, p<0.001). Diagnostic accuracy for NBM 200 for anaemia was as follows: sensitivity 33.7% (95% CI 27.3% to 40.5%), specificity 91.8% (95% CI 81.9% to 97.3%), PPV 93.3% (95% CI 85.1% to 97.8%), and NPV 28.9% (95% CI 22.6% to 35.8%). The ICC was 0.22 (95% CI −0.08 to 0.47) for an absolute agreement.
Bland-Altman Analysis of Sysmex XP-100 and NBM 200 haemoglobin measurements. *ULA, upper limit of agreement; LLA, lower limit of agreement. LLA −4.79 g/dL and ULA 1.01 g/dL, mean difference −1.8 g/dL (95% CI −2.06 to −1.71).
Discussion
In this study involving women during pregnancy, we found that Hb measurements obtained from NBM 200 were generally higher than the measurements obtained from Sysmex XP-100 (gold standard). Consequently, NBM 200 grossly underestimated the anaemia prevalence in our study population (sensitivity of 33.7%). The ICC indicated fair agreement for NBM 200; however, we identified possible patient safety concerns with the use of NBM 200, considering that all the severe anaemia cases in our study participants were misclassified as having moderate anaemia and would not have received appropriate clinical intervention had this method alone been used (table 1).
Our study has many strengths; to the best of our knowledge, it is the first study where prospectively collected data are presented from a large representative population of pregnant women in a rural community setting.4 The study population was drawn from marginalised and difficult to access areas where comparatively few healthcare facilities are available, and thus evaluating non-invasive portable technology in geographically remote areas is highly important. Second, our study recorded a good response rate (96.8%), with only seven eligible women declining to participate. Lastly, the non-invasive readings were obtained in accordance with the manufacturer's guidelines, and none of our subjects had thumb deformity, infection, ulcerations, oedema or skin colourants such as henna (locally known as Mehndi).12 However, a technical failure of the non-invasive sensor occurred twice during the study, which delayed data collection, and caused the loss of two samples as mentioned before.
Our study findings are in agreement with those from a study conducted at a blood centre in Seoul, Korea by Kim et al13 where the NBM 200 Hb measurements tended to be higher than the LH500 automated haematology analyser estimates (Beckman Coulter Inc., Brea, California, USA). The sensitivity (38.6%) and specificity (93.6%) analyses were very similar to our study and indicated that NBM 200 failed to detect more than half of the ineligible blood donors. Though the correlation between these two techniques was satisfactory (rs 0.86), the strong agreement may not be the criteria for donor selection, as accurate blood parameters are prerequisites to prevent any ineligible donor selection. Similarly, an Italian study (n=3995 donors) showed a low sensitivity using NBM 200 (36.03% in the men's group and 45.76% among women) on comparison with the gold standard Beckman Coulter's AcT-5 diff AL (Beckman Coulter Inc., Brea, California, USA).14 However, our findings are in contrast to a recent study involving blood donors (n=485, 94% men) from North India that reported a 71.7% sensitivity for NBM 200 when compared with the Sysmex KX-21 analyser (Sysmex Corporation, Kobe, Japan) (table 2).5 Despite the high sensitivity, the Bland-Altman limits of agreement were wide (Upper limit of agreement: 2.09 and Lower limit of agreement: −3.39) with poor correlation (rs=0.43) between the two Hb estimation methods. About 45.5% of ineligible donors were not detected in the Indian study,5 similar to the study by Kim et al.13 A second study from Northwest India6 involving 200 blood donors (96% men) showed high sensitivity (96.36%) when compared with the Sysmex KX-21 analyser (Sysmex America Inc., Lincolnshire). The results by NBM 200 showed wide variation on assessing correlation with the gold standard; however, the mean of difference of two measurements was non-significant.6 Belardinelli et al15 also reported high sensitivity (98%) and specificity (97%) in a similar study population. It is worth noting, however, that all the patients in their study had Hb values above 13 g/dL (as ascertained using the Beckman Coulter AcT-5 diff cell counter) and are unlikely to be representative of the general population.
Published studies conducted to validate the NBM 200 sensor
A study8 involving pregnant women from Israel (n=63) reported Hb in the range 6.9–13.9 g/dL by the gold standard (LH750 analyser, Beckman Coulter Inc., Brea, California, USA), which was lower than the range reported by NBM 200 (7.7–14 g/dL). The Bland-Altman analysis showed wide limits of agreement (−1.59 to 1.79) with an SD error of 0.86 g/dL. The study concluded that the sensor measures Hb accurately mainly based on a strong correlation; however, correlation measures association and not agreement.11 ,13 Second, the range of Hb values obtained by the gold standard and NBM 200 clearly indicate that NBM 200 missed some severe anaemia cases, similar to our experience.8 Lastly, the study involved pregnant women from all trimesters (gestational age: average 35.9 weeks, range 13–41 weeks), where Hb is likely to vary,9 while our findings are based on a larger sample size (n=269) between a fixed gestational period (3 and 5 months of pregnancy) and included a more detailed diagnostic analysis reporting the Bland Altman agreement method, sensitivity, specificity, NPV, PPV and correlation analyses.
Previously published studies of the NBM 200 sensor (table 2) have been mostly conducted in prospective blood donors,5 ,6 ,12 ,14 ,15 in whom Hb is likely to be higher than in the general population. Moreover, the two Indian studies outlined earlier had fewer than 7% women participants.5 ,6 A report from the Indian National Family Health Survey 2005–2006 showed a 24% anaemia prevalence in men (aged 15–49 years), which was much lower than in women (55% prevalence, 15–49 years).16 On the basis of our results, NBM 200 underestimates anaemia prevalence; therefore, the validation of the non-invasive technology predominantly in male blood donors could explain the different findings. The study by Singh et al5 had a prevalence of 2.3% anaemic cases identified by the gold standard, and the study by Malukani et al6 had only 17% anaemic cases as compared to our study with 77.3% anaemic cases, suggesting sampling variability.4 Studies from Italy, Germany and France involved a fairly equal number of men and women reporting to blood donation centres, but anaemia prevalence is much lower in these countries compared to India. The German study reported a mean Hb value of 13.4 g/dL (SD 0.93) in women (using the gold standard, Sysmex KX-21),12 while in our study settings in rural India the mean Hb was 10.0 g/dL (using the gold standard, Sysmex XP-100). Similarly, the mean Hb in the French study was 13.2 g/dL (95% CI 11.9 to 14.3),1 and in the Italian study the boxplot indicated a mean Hb of 14.0 g/dL.15 A systematic review and meta-analysis by Kim et al4 of non-invasive Hb sensor technologies (Radical 7, NBM 200, Pronto 7 and NBM 200MP) found that the pooled mean difference and SD were 0.10,±1.37 g/dL, respectively (95% CI −2.59 to 2.80, I2=95.9% for mean difference and I2=95.0% for SD). The review concluded that, owing to the wide limits of agreement with reference methods, clinical decisions based on non-invasive devices should be made cautiously. All studies included in the review were hospital-based, and none of the studies were from developing countries where anaemia prevalence is typically much higher.
Conclusions and implications
This is the first study conducted in a rural community setting where a representative sample of pregnant women was assessed. Our measurements were obtained in the field during a household survey which provided a unique opportunity to test the non-invasive technology in rural areas, where diagnostic facilities are limited. Globally, anaemia is a major public health issue affecting pregnant women, particularly in a developing country like India. Laboratory investigation of anaemia requires established infrastructure and the facility to transport blood samples. A portable non-invasive sensor that provides rapid results could provide opportunities for population level anaemia screening; however, the technology needs to be improved substantially to provide accurate readings in line with the accepted gold standard, before it can be implemented in a rural Indian population. Since most studies, including our own, suggest a systematic overestimation of Hb values by NBM 200, it may be possible for the manufacturer to improve the performance by recalibrating the sensor or algorithms that process the data collected. If the melanin content of subjects is demonstrated to interfere with Hb readings, then this would need further consideration. In future, field-based research in developing countries following improvements to the sensor is required, before advocating the adoption of non-invasive technology for Hb measurement and anaemia diagnosis.
Acknowledgments
The authors acknowledge the support of Ms. Sandhya Rankhamb in data collection, data entry, verification, and recognise her contribution in the project. The authors thank HMF village health workers for providing field level support for this study. The authors dedicate our research to Dr Sulbha Hardikar and Professor (Mr) and Mrs Chawathe who supported the project and PhD studies, respectively.
Footnotes
Twitter Follow Anand Ahankari at @AnandAhankari
Contributors The study was designed by AWF, PRM, LJT and ASA. ASA obtained the data and ASA, PRM and AWF conducted the analysis. All authors (PRM, LJT, JVD, ASA and AWF) participated in manuscript preparation and approved the manuscript for publication.
Funding The study was part of a postgraduate research programme of ASA with the University of Nottingham, UK. The PhD study was sponsored by the Vice Chancellor Scholarship for Research Excellence 2013 granted by the University of Nottingham, UK (Ref 12031). The project was implemented in India as a joint collaboration with the Halo Medical Foundation, which was supported by Dr Hardikar through the Maharashtra Foundation, USA. The non-invasive NBM 200 unit was sponsored by the Clinical Translational Research Priority Group Award for International Collaboration, University of Nottingham, UK (Grant Code: A2RN72). Professor (Mr) and Mrs Chawathe, Mumbai, India provided generous support for ASA's study. ASA also received a bursary from Durgadevi Charitable Trust, India during the PhD programme.
Competing interests None declared.
Ethics approval (1) Institutional Ethics Committee, Government Medical College, Aurangabad, India. (2) Medical School Ethics Committee, University of Nottingham, Nottingham, UK.
Provenance and peer review Not commissioned; externally peer reviewed.