Article Text
Abstract
Clinicians have historically been integral in innovating and developing technology in medicine and surgery. In recent years, however, in an increasingly complex healthcare system, a doctor with innovative ideas is often left behind. Transition from idea to bedside now entails significant hurdles, which often go unrecognised at the outset, particularly for first-time innovators. The BioInnnovate Ireland process, based on the Stanford Biodesign Programme (Identify, Invent and Implement), aims to streamline the process of innovation within the MedTech sector. These programmes focus on needs-based innovation and enable multidisciplinary teams to innovate and collaborate more succinctly. In this preliminary study, the authors aimed to examine the impact of BioInnovate Ireland has had on the clinicians involved and validate the collaborative process. To date, 13 fellows with backgrounds in clinical medicine have participated in the BioInnovate programme. Ten of these clinicians remain involved in clinical innovation projects with four of these working on Enterprise Ireland funded commercialisation grants and one working as chief executive officer of a service-led start-up, Strive. Of these, five also remain engaged in clinical practice on a full or part-time basis. The clinicians who have returned to full-time clinical practice have used the process and learning of the programme to influence their individual clinical areas and actively seek innovative solutions to meet clinical challenges. Clinicians, in particular, describe gaining value from the BioInnovate programme in areas of ‘Understanding Entrepreneurship’ and ‘Business Strategy’. Further study is needed into the quantitative impact on the ecosystem and impact to other stakeholders.
- innovation
- education
- collaboration
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
‘A new type of thinking is essential if mankind is to survive and move toward higher levels.’
Albert Einstein1
‘Innovation’ is a buzz word that often brings to mind the explosion of internet technologies and advances in telecommunications over the last 20 years. The Organization for Economic Cooperation and Development (OECD), however, defines innovation as going ‘beyond R&D. It goes far beyond the confines of research labs to users, suppliers and consumers everywhere—in government, business and non-profit organisations, across borders, across sectors, and across institutions.’2 In addition, the focus of innovation must move beyond traditional research and focus on the implementation and adoption of new techniques and services.
The term innovation is now being increasingly used to describe changes and advances in the delivery of healthcare. The translation of innovative solution into frontline healthcare outcomes, however, comes with many hurdles which often go unrecognised at the outset, in particular to first-time inventors and entrepreneurs. Successful adoption of a novel technology, device or pharmaceutical can be dictated by an entrepreneur’s ability to identify and navigate through challenges relating to regulatory bodies, reimbursement pathways and clinical barriers to entry. Implementation strategies to mitigate these risks are critical to the journey, as a product goes from ‘bench to bedside’. Concurrently, an ageing population has led to a significant increase in unmet clinical needs. This has been coupled with rapidly rising healthcare costs and increasing societal expectations of healthcare delivery. These factors are contributing to the challenges in sustainability of the current healthcare systems. Given these complexities, collaborative innovation is imperative to reduce the time and cost of developing new solutions and delivering them to patients. Working as a team allows individuals bring together their ‘skills and ideas from disparate areas to produce something new.’3 This approach is a cornerstone of the BioInnovate process (figure 1).4
BioInnovate placed at the Interface of Industry, Academia and Clinical Medicine.
In 2001, Stanford University responded to these complexities by establishing the multidisciplinary programme ‘Biodesign’—‘to promote education and mentoring in the area of biomedical technology innovation.’5 Based on the success of this programme, the BioInnovate Ireland Fellowship has been established over the last 5 years to facilitate the development of new ideas and solutions within the MedTech sector in Ireland. BioInnovate is positioned as a ‘medical technology innovation training programme that aims to act as a neutral territory in which academia, clinicians and industry can collaborate to develop novel medical technologies.’4 The Fellowship has been modelled on, and is affiliated with, the Stanford Biodesign Programme in focusing on needs-based innovation.6 The programme facilitates the collaboration and development of entrepreneurship in the Medical Technology sector in Ireland.7 The core basis of the programme lies in the recruitment of fellows from diverse backgrounds including, but not exclusively, clinical medicine, biomedical engineering, chemical engineering, pharmaceuticals, business development, industrial design and project management. Each team has four members with a minimum of one clinician on every team to provide clinical insights and guide members of the team without prior clinical knowledge in understanding disease states and clinical pathways.
The process focuses on identifying unmet clinical needs that have the potential to be solved with the use of an innovative medical technology solution. The process follows a clear outline of three distinct phases,8 identification of needs, invention of concepts surrounding that need and, finally, implementation and translation of a solution to the bedside. Throughout the process, there is a focus on the creation of value and continuously analysing the value proposition of the innovation within the marketplace.
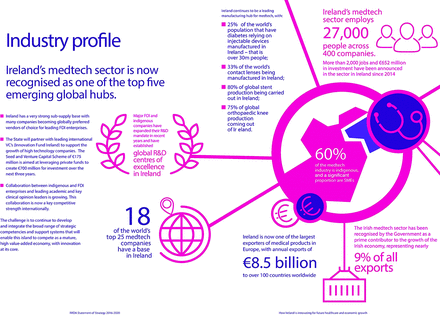
Ireland currently plays a substantial role in the MedTech industry with a significant presence of the leading MedTech companies with operations based in Ireland. Currently, 27 000 people are employed in the sector across 400 companies with direct exports to over 100 countries with a value in excess of €9 billion. There are a number of identifiable factors that contribute to Ireland’s competitive position (figure 2).9 These include world-class technical and managerial talent, competitive corporation tax rates and various programmes that encourage knowledge development and innovation. Ireland has also been identified as a location that has significant product development expertise as well as efficient collaboration across diverse disciplines and a history of manufacturing excellence.9 This environment has led to world leaders in the medical device industry valuing Ireland’s contribution to the sector, with companies such as Stryker, Medtronic and Cook Medical opening research and development facilities to leverage local capabilities in medical device design and development.10 Concurrently, there are a growing number of MedTech start-ups based in Ireland. This is due in no small part to the need for economic diversity following global economic instability and is also a reflection of the high level of education and skills that are available within our small island.
R&D, research and development. FDI, Foreign Direct Investment; VC, venture capital; SME, Small to Medium Enterprise.
With close relationships fostered with academia, industry, clinicians, investors and the internationally acclaimed, Stanford Biodesign Programme, BioInnovate has developed a microecosystem within the Irish MedTech industry. This is strongly supported by the highly experienced members of the advisory board and mentors who have experience in MedTech early investment, strategic investment as well as clinical and regulatory strategy.
Clinical innovation and entrepreneurship
Medical practitioners have always played an integral part in innovation in medicine. Both in the development of new techniques and the addition of various tools and technologies to their armour, doctors have often pushed boundaries in order to improve patient care. Creativity was an integral part of the formative years of modern medicine. Complex healthcare systems, however, pose a challenge to those wishing to create novel solutions at the bedside. The transition from idea to bedside now comes with significant hurdles which must be overcome.
Emerging programmes, such as BioInnovate and Stanford Biodesign, can allow creative and innovative doctors to gain the tools to address these new complexities. In modern clinical training, the focus of postgraduate research and career progression is often focused on biomedical research, clinical research and education. Value, however, is now evident in non-traditional educational activities that encourage collaboration across disciplines and development of skills in medical innovation. Clinicians also need to be active members of medical device innovation teams as the essential knowledge of medical practice is crucial to effective development of technologies.11 A special report by the American Heart Association in 2015 outlined the key value that can be created by fostering the training and careers of clinical innovators. In line with the rapid growth in technology and ‘big-data’, there is now a need for agility in the healthcare system in particular in educating future clinicians.12 Furthermore, changing focus of healthcare systems towards affordability and outcome measures offers an opportunity for clinicians to become involved in truly disruptive innovations. Convergence of technologies and disciplines, in particular in the area of connected health and mobile technology, can be positively impacted by the involvement of first-line caregivers. Members of the clinical community have unique, hands-on experience of the challenges and unmet needs within the healthcare system that can lead to the development of new ideas, technologies and services. Despite the complexities, MedTech companies remain reliant on the essential knowledge of technical and medical aspects that can influence product development from concept to delivery.11 Programmes such as BioInnovate Ireland and Stanford Biodesign, which allow clinicians to more effectively work in collaboration with industry and MedTech start-ups, can encourage positive outputs and developments across multiple arenas. The prominent feature of these programmes is the focus on ‘needs-based’ innovation which forces the innovators to focus on clinical needs directly observed in varying clinical areas. This has proven to be an extremely successful method of bringing concepts from idea to bedside in a relatively timely fashion.8
Methods
In this small study, the authors aimed to quantify the impact that 5 years of BioInnovate Ireland has had on the clinicians involved, validate the collaborative process and ultimately learn from their experiences. To date, 13 fellows with a background in clinical medicine have completed the BioInnovate Fellowship. Each of the 13 clinicians approached the programme from varying backgrounds in general medicine, emergency medicine, general practice, surgery, radiology, and anaesthesia and critical care.
Following discussions with clinical director and programme manager of BioInnovate, a questionnaire was developed to answer specific questions regarding the motivations and outcomes for clinicians who have taken part in BioInnovate Ireland to date.
The following is the list of questions:
Why did you decide to do BioInnovate? (multiple responses allowed)
Frustrated with clinical medicine
Looking for research opportunities
Interested in a start-up
Unsure of career progression
Other (please specify)
What were your expectations of BioInnovate?
Were you involved in MedTech innovation prior to BioInnovate?
How much knowledge of the industry did you have?
A lot—previous engagement
Some—knowledge based on personal interest
None—entered the programme thinking it would be of interest
What skills do you think this programme impacted? (1=no impact, 5=significant impact)
Communication
Understanding entrepreneurship
Business strategy
Teamwork
Critical analysis
Have you returned to clinical practice?
If yes, do you still engage in clinical innovation projects?
If yes, has the programme impacted how you practice medicine?
If no, are you still working on a BioInnovate-based project?
Were the expectations of the programme met?
Each clinician was emailed and requested to respond to the survey using an anonymous online survey collection facility. Results were reviewed and collated.
Results
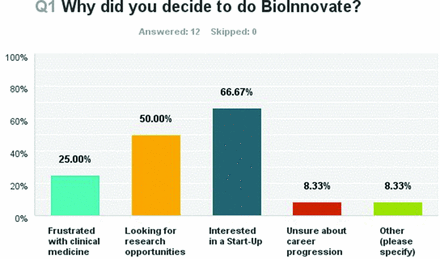
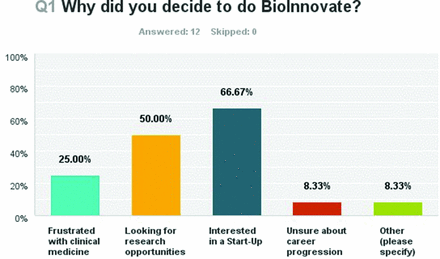
Twelve of the 13 clinicians responded to the survey, giving a 92.3% response rate. Of these, 66.67% (eight respondents) were interested in pursuing a MedTech start-up, 50% were looking for research opportunities, 25% were frustrated with clinical medicine and 8.33% were unsure of career progression. One respondent was looking for a ‘fresh perspective on Medical Device Sphere’ (figure 3). The majority of respondents (91%) had no previous involvement with Medical Technology prior to embarking on the fellowship. One respondent had previously worked in the MedTech industry while the majority (58.33%) had some knowledge of the industry based on personal interest while the remainder (33.33%) entered the programme thinking it would be of interest to them. Knowledge of the MedTech industry was also varying, with the majority (58%) reporting some knowledge based purely on personal interest. Thirty-three per cent admitted no previous knowledge of the sector. Skills which respondents subjectively reported improvement in were also identified. Areas where the programme had the most impact were ‘Understanding Entrepreneurship’ and ‘Business Strategy’, with 75% of respondents reporting moderate or significant impact on these skills. In addition to this, 7 of the 12 respondents reported moderate or significant impact on teamwork and critical analysis skills.
Question One of Survey-Why did you decide to do BioInnovate Fellowship?
Reported expectations of the programme varied from ‘expand my professional network’ to ‘possibly get a patent’. The majority of respondents described interest in the process and multidisciplinary aspects of the BioInnovate programme, in particular the introduction to engineers and business experts. One clinician stated, ‘I expected it to provide an environment more conducive to innovative thinking than would be the case with the routine clinical environment which can often be guide line driven rather than thinking outside the box.’ Others were simply interested in gaining a higher degree, in particular a Master of Science. Some criticisms that emerged from the survey included the significant focus on medical devices and limited scope for other healthcare innovations—‘little time give to innovation form a medical IT or connected health point of view.’ One respondent felt that there was limited reciprocity of knowledge once the clinicians had given their input—‘No reciprocal understanding of business aspects of innovation or details of engineering aspects provided by team unless heavily asked for. Heavy reliance on the clinician until idea has been generated and then relatively poor engagement thereafter.’ In addition, criticisms of team dynamic management emerged due to the development of strategic alliances between two opposing teams, thus effectively excluding the clinician from further involvement in a subsequent start-up venture. The majority of clinicians felt that the programme either somewhat or totally met their expectations (figure 4). Five respondents have returned to full-time clinical practice while three have returned part-time and four have not returned. One respondent described surprise at not returning to clinical practice—‘I came into the program expecting to leave with an MSc (Masters of Science) and never look back. During the programme, my point of view changed and I have now left medicine and am pursuing a Medical Device project.’ Six of the respondents feel that the process of BioInnovate has somewhat changed their practice of medicine with one respondent commented, ‘it certainly encourages you to challenge the status quo in clinical practice, and also because I am subspecialising in interventional radiology which relies on minimally invasive devices, whereby thinking outside the box is regularly called on.’ Benefits of the ‘Identify’ stage of the process were particularly highlighted by one respondent. ‘It was great to learn problem identification and how to process the mountain of information in an efficient manner and to learn the various necessary steps and time and hard work that takes place before an unmet clinical need is translated into a medical device that can benefit patients. In my opinion, the programme met this expectation.’
Final Question-Where your expectations met during the programme?
Ten of the clinicians remain involved in clinical innovation projects with four of these working on Enterprise Ireland funded commercialisation grants and one working as chief executive officer of a service-led start-up, Strive. Three clinicians have also been finalists in the Irish Medical Device Association/Cleveland Clinic Innovation awards with two winners and two of the clinicians continue to actively collaborate with the Mayo Clinic as part of their projects.
Discussion
The above study displays that clinicians have gained significant experience and value from the BioInnovate programme. Opportunities to diversify their clinical practice and become involved in start-up projects have a positive impact on the MedTech ecosystem in Ireland. Previously, start-ups have relied on expertise from the USA to advise the development of strategy; however, with the dissemination of knowledge throughout communities where programmes such as BioInnovate are located, a competitive edge can be gained in this space. In addition, clinicians returning to clinical practice will facilitate the expanding network and create further opportunities for the development of ideas.
While practising medicine, clinicians often take for granted the tools and technologies that are at our fingertips. From initial days as medical students, we use medical devices like cannulas, catheters, ECG machines, ultrasound machines and temperature monitors without question. In fact, we do not question how the technology got there, and we expect the technology to advance as quickly as the technology we use in our daily lives. Insights, and education in this area, are distinctly lacking in the traditional medical school model. This is in contrast to the significant focus on entrepreneurship and innovation in other disciplines. Skills in need of identification, clinical trial design, regulatory pathways, reimbursement challenges and strategic development will also positively impact the clinical setting within Ireland. As demonstrated by the survey, many clinicians will return to clinical practice following the programme and may not be actively involved in a project that has stemmed directly from it. Despite this, their skills within the workforce will enhance the ecosystem and ultimately patient care. Recommendations for the success of clinician innovators include rewarding contributions to clinical innovations in a similar manner to traditional research, including funding for commercialisation from government bodies, angel or venture capital investment in projects, regulatory approval for products and return on investment. In centres such as the Mayo Clinic, clinicians may use allocated time towards innovation projects akin to traditional research time, which can ultimately become profitable for the clinician’s department if innovations are successful.13
Ethical consideration
As growth occurs within the field of clinician innovation, and increasing numbers of doctors wish to engage in entrepreneurial activities, ethical questions may occur. Moral principles uniquely assigned to the medical profession can be challenged when in pursuit of entrepreneurial activities which may be seen to be compromised.14 Importantly, however, there are moral obligations to improve the health of individual patients and society as a whole. Innovative products can have the ability to ultimately alter population health and change the course of disease states for many millions of patients. The cardiovascular space has demonstrated this over the last 50 years and much of the work of clinician innovators in cardiology is defining how other specialties progress.
In a modern context, the cost of developing a high-quality product or service that can fulfil the regulatory and safety criteria that govern modern practice is very high in contrast to the ‘kitchen-table’ approach often described in the past. This change, therefore, requires significant investment from other interested parties such as venture capital and angel investors. These investors are unlikely to adopt a product if they do not see a return on investment through licensing or sales. The polarised goals of clinicians and investors can therefore generate a significant conflict of interest (COI). The juxtaposition of values can be challenging for clinician innovators and it is their responsibility to identify ethical concerns and COIs at the outset in order to maintain the public’s trust.
COIs are often cited as a significant challenge to collaboration with industry and the development of innovations. Donovan and Kaplan14 identify areas within the innovation process where COI challenges may occur. These include ‘insuring safety and best care’, ‘protocol development’, ‘patient-screening and consent’, ‘performing the procedure’ and ‘data management’. They have suggested that in order to mitigate the risks of COI, patient care decisions should not be undertaken by the clinician innovator and ultimate responsibility of care should lie with another senior clinician. Other recommendations include limiting the clinician entrepreneur’s role to technical aspects and developing a management plan for prospective COIs that may evolve. Ultimately, if a balance is struck, the goal of improving patient care can be achieved in an ethical and fair manner.
The Irish context
Ireland, similar to some of the major ecosystems in the USA such as Silicon Valley and Boston, has the capability to continue to nurture the development of clinician innovators and collaborators in the development of health technologies. It is imperative, however, to gain engagement from the wider clinical community and establish a support network and recognition of the experience gained from programmes like BioInnovate within specialty training schemes. As outlined, Ireland has been playing a growing part in the research, development and manufacturing of medical devices. Ireland is uniquely positioned in the MedTech industry despite its small size. From an academic point of view, we boast 45 higher education institutions that provide the highest proportion of science and engineering graduates in the OECD. We also have six medical schools where over 1000 doctors graduate per year. Recently, a number of high-quality clinical research facilities have opened which will continue to allow Ireland become a location of world leadership in medical technology delivery. In order to maintain and grow this position, continuing support is needed for the development and adoption of innovative advances in medical delivery. Supporting clinicians in pursuit of a non-traditional career pathway will encourage the development of the ecosystem in Ireland. Currently, programmes in medical innovation are not recognised by the postgraduate training bodies as part of specialist training. This, therefore, limits the number of candidates willing to engage in such activities. In particular, it impedes candidates who have already begun specialised training to engage in innovation activities during their training. This is reflected in the recruitment to BioInnovate to date with clinicians either having completed their specialist training or not commenced higher specialist training. There is significant benefit to be gained by engaging clinicians in specialist training as they will be intimately familiar with the problems and challenges within their subspecialties.
Furthermore, the growing number of postgraduate entrants to Irish medical schools will have a positive impact on the ability of our healthcare system to collaborate and innovate while leveraging skills learnt in undergraduate programmes. Steps to encourage clinicians to use creative thinking and divergence of skills are imperative in order to accelerate change.
Conclusion
This small study supports the position of BioInnovate Ireland as a facilitator of innovation that lies at the cross section of clinical, academic and industrial practices. As demonstrated in the results of the survey, the majority of clinicians who commenced the programme had little or no knowledge of the MedTech sector. Following completion, 10 of the 12 respondents remain actively engaged in innovative projects in the MedTech and healthcare delivery sector.
In addition, recently published data from Stanford Biodesign15 suggest that there is an overall positive impact of their programme on the career trajectory of their fellows. Similarities can be drawn between their analysis and that in BioInnovate Ireland regarding the impact on business strategy skills and teamwork; however, further study of the non-clinical fellows would be required to allow accurate comparisons to be made.
Programmes such as this can facilitate education in the area of entrepreneurship and innovation can support the ongoing development of the MedTech community in Ireland. Additional fellowship teams focusing on connected health and digital medical technology have been now introduced into the BioInnovate programme demonstrating the development of a dynamic fellowship which has the ability to respond to changes in the clinical needs. Ongoing challenges in maintaining ethical and moral standards as well as appropriate clinical engagement must be consistently addressed. The programme does serve to improve the strength and depth of the local ecosystem as well as ultimately identify and solve needs which will lead to improved patient care.
BioInnovate Ireland has become a facilitator of collaboration at the cross section of clinical, academic and industrial practices; however, further research is required to establish the impact on other stakeholders within the BioInnovate teams, as well research into the quantifiable impact which the programme provides on the surrounding ecosystem as a whole.
Acknowledgments
This work was supported by all the staff of BioInnovate Ireland.
Footnotes
Contributors The majority of this work has been undertaken by the lead author, EKMG. Development of questionnaire was facilitated by PA and FS. Access to respondents was provided by BioInnovate Ireland database. Editorial and formatting were facilitated by IK.
Funding The primary author is a former fellow of BioInnovate Ireland which is funded by Enterprise Ireland. This research is not tied to any specific current or future funding grants.
Competing interests EKMG is a former fellow of BioInnovate Ireland which is funded in part by Enterprise Ireland. FS and PA currently direct and manage BioInnovate Ireland which is facilitated by the National University of Ireland, Galway.
Provenance and peer review Not commissioned; externally peer reviewed.